The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
The latent heat of vapourisation of water at 100 Celcius is 540 cal/g . Calculate the entropy increase when one mole of water at 100 Celcius is evaporated.

⏩SOLVED:Calculate the entropy change for the conversion of…

559) Calculate the entropy change when 3.6 g of liquid water is completely converted into vanours 373 K. The molar heat of vaporization of water is 40.85 kJ mol! b) 2.189 JK

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 Kis: (specific heat of water liquid and water vapour are 4.2 kJ K-kg- and

SOLVED: Calculate the entropy change corresponding to the process of vaporization of 1 mol of liquid water at 0°C and 1 atm into steam at 100°C if the process is carried out

Calculate the change in entropy when 40g of water at 50 C are mixed with 80 g of water at - Chemistry - Thermodynamics - 16219541
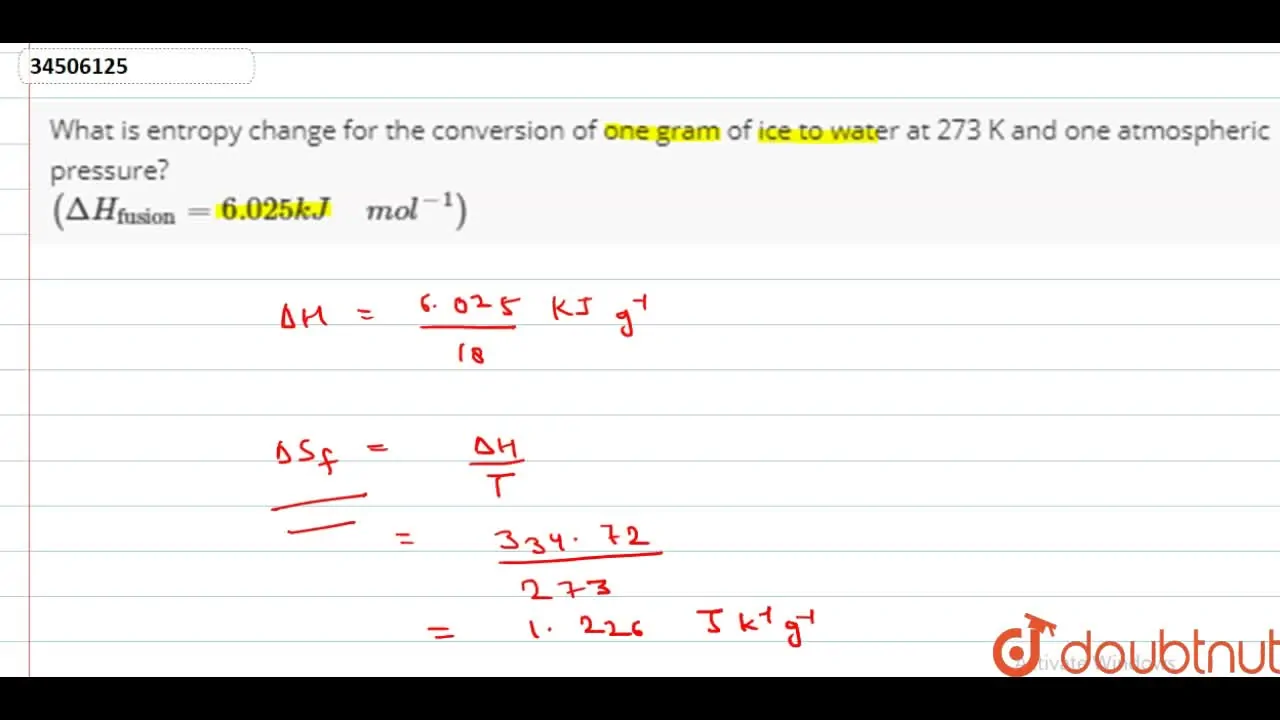
What is entropy change for the conversion of one gram of ice to water

The entropy change when 36g of water evaporates at 373 K is :- (DeltaH
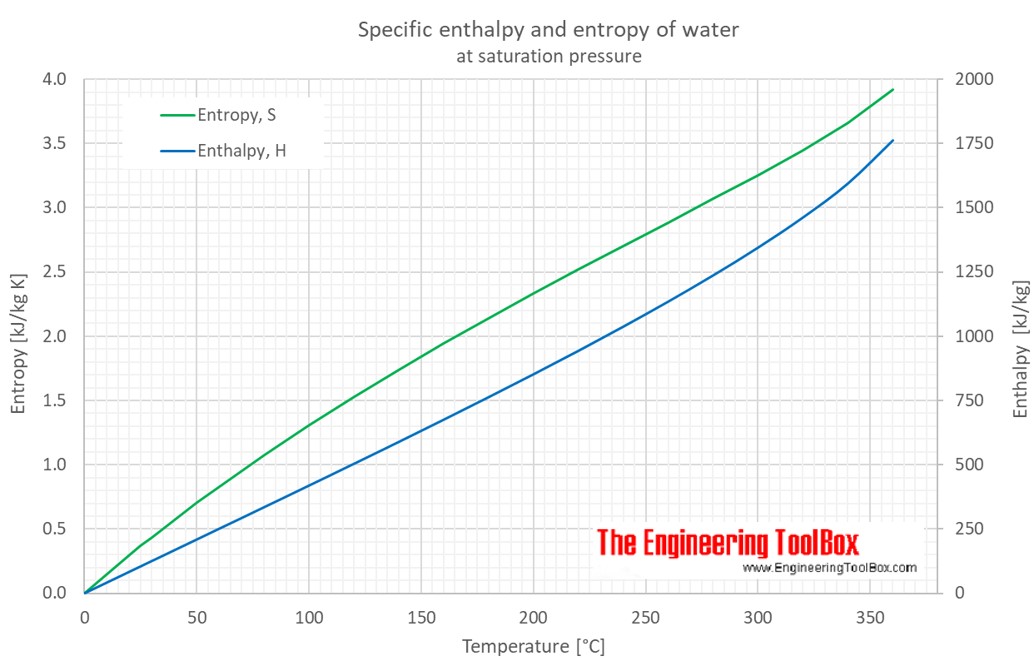
Water - Enthalpy and Entropy vs. Temperature

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is

The entropy change when `36g` of water evaporates at `373 K` is `:-` `(DeltaH=40.63(KJ)/(mol))`

Hybrid solar evaporation system for water and electricity co-generation: Comprehensive utilization of solar and water energy - ScienceDirect

to insulated co 9.0 g ice O'C is mixed with 36 g of water 50°C in a thermally data, answer the question that follow ? Comprehension Q.8 Th rea 8 Final temperature

Calculate the entropy change involved in the vaporisation of water at
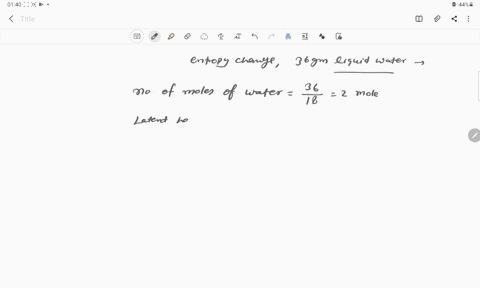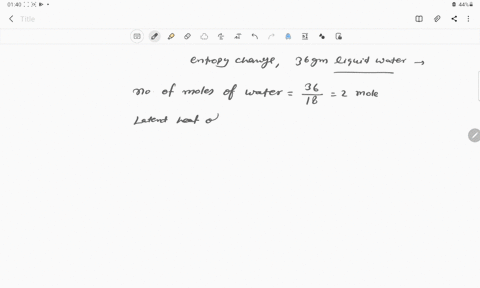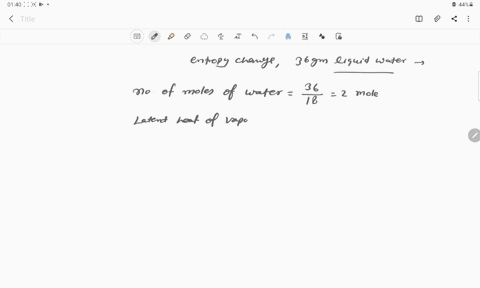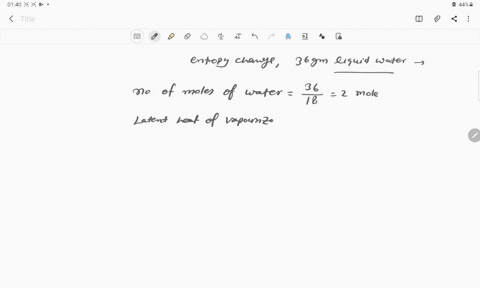
⏩SOLVED:Calculate the entropy change for the conversion of…







