Solved] Why is the compressibility factor less than 1 at most
Description
Answer to Why is the compressibility factor less than 1 at most conditions?

The compressibility factor of a gas is less than 1 at STP. Its

Compressibility of Liquids - an overview

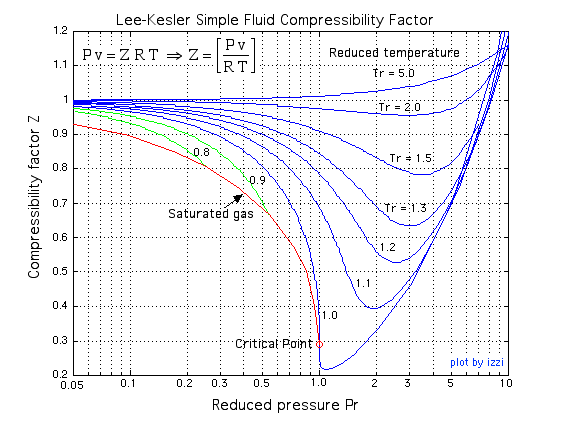
The compressibility factor Z a low-pressure range of all gases

1.7: Connecting the van der Waals and the viral equations- the

Compressibility Factor Z Important Concepts and Tips for JEE Main
Why compressibility factor of areal gas is greater than unity at
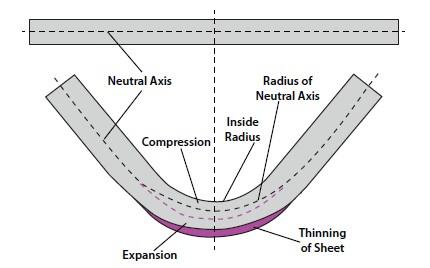
Analyzing the k-factor in sheet metal bending
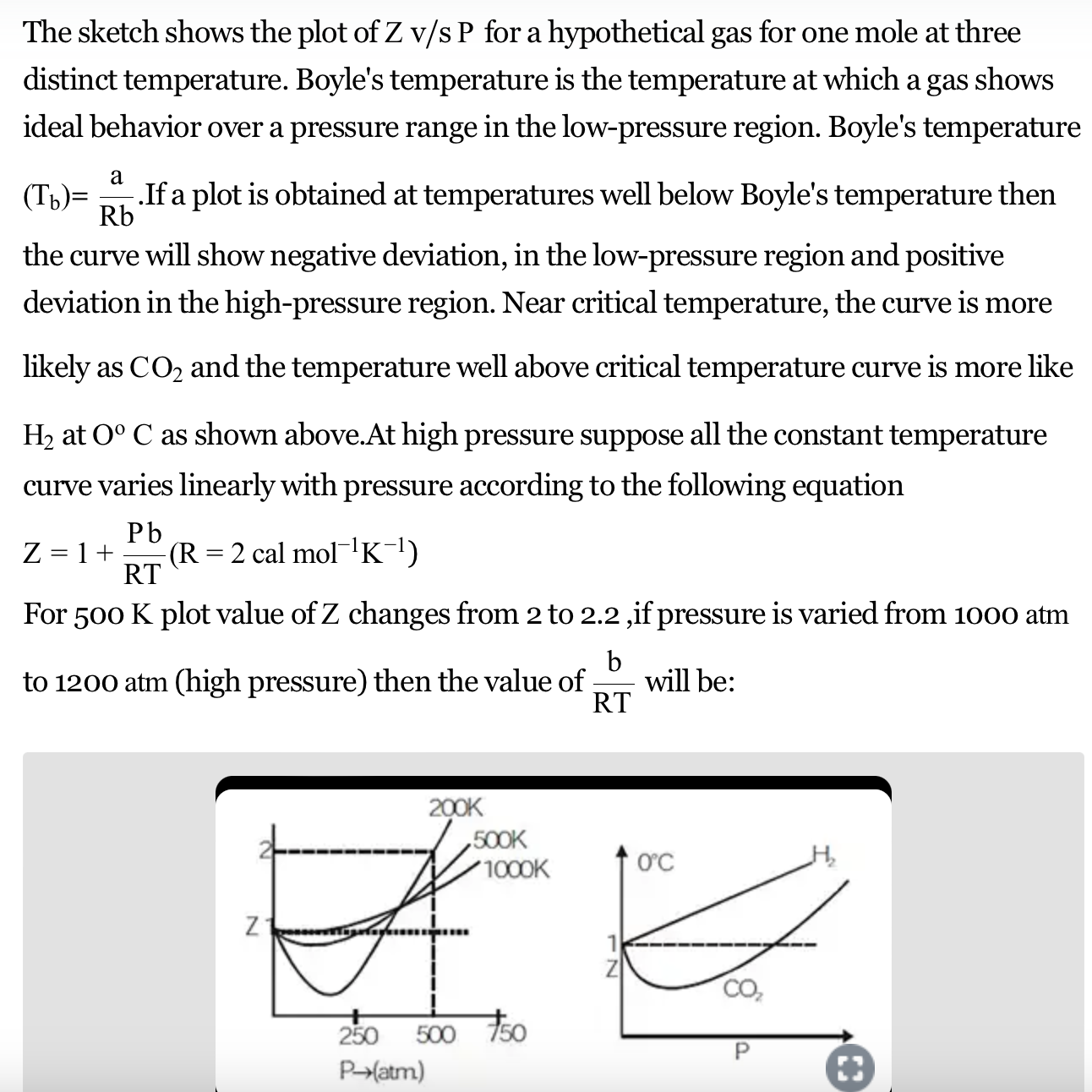
Solved The graph of compressibility factor (Z)v/sP for 1 mol

Deviation Of Real Gas From Ideal Gas Behavior

Solved True or false: If the compressibility factor (z) is
Why compressibility factor of areal gas is greater than unity at
Related products
$ 16.99USD
Score 4.6(120)
In stock
Continue to book
$ 16.99USD
Score 4.6(120)
In stock
Continue to book
©2018-2024, followfire.info, Inc. or its affiliates