Protocol for a randomised controlled feasibility trial of exercise
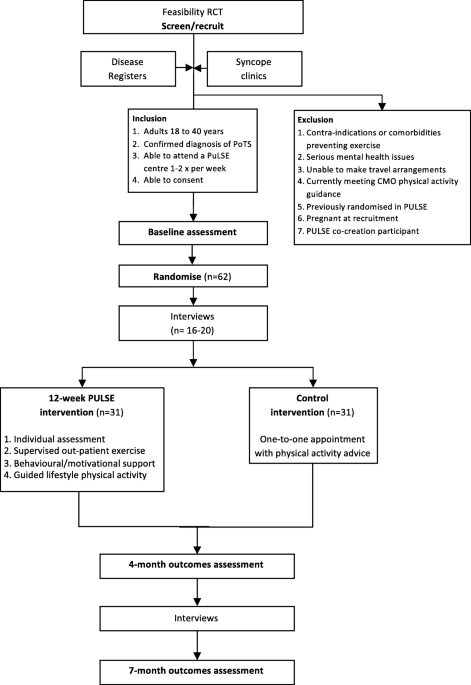
Background Postural orthostatic tachycardia syndrome (POTS) is an autonomic nervous system disorder causing an abnormal cardiovascular response to upright posture. It affects around 0.2% of the population, most commonly women aged 13 to 50 years. POTS can be debilitating; prolonged episodes of pre-syncope and fatigue can severely affect activities of daily living and health-related quality of life (HRQoL). Medical treatment is limited and not supported by randomised controlled trial (RCT) evidence. Lifestyle interventions are first-line treatment, including increased fluid and salt intake, compression tights and isometric counter-pressure manoeuvres to prevent fainting. Observational studies and small RCTs suggest exercise training may improve symptoms and HRQoL in POTS, but evidence quality is low. Methods Sixty-two people (aged 18–40 years) with a confirmed diagnosis of POTS will be invited to enrol on a feasibility RCT with embedded qualitative study. The primary outcome will be feasibility; process-related measures will include the number of people eligible, recruited, randomised and withdrawn, along with indicators of exercise programme adherence and acceptability. Secondary physiological, clinical and health-related outcomes including sub-maximal recumbent bike exercise test, active stand test and HRQoL will be measured at 4 and 7 months post-randomisation by researchers blinded to treatment allocation. The PostUraL tachycardia Syndrome Exercise (PULSE) intervention consists of (1) individual assessment; (2) 12-week, once to twice-weekly, supervised out-patient exercise training; (3) behavioural and motivational support; and (4) guided lifestyle physical activity. The control intervention will be best-practice usual care with a single 30-min, one-to-one practitioner appointment, and general advice on safe and effective physical activity. For the embedded qualitative study, participants (n = 10 intervention, n = 10 control) will be interviewed at baseline and 4 months post-randomisation to assess acceptability and the feasibility of progressing to a definitive trial. Discussion There is very little high-quality research investigating exercise rehabilitation for people with POTS. The PULSE study will be the first randomised trial to assess the feasibility of conducting a definitive multicentre RCT testing supervised exercise rehabilitation with behavioural and motivational support, compared to best-practice usual care, for people with POTS. Trial registration ISRCTN45323485 registered on 7 April 2020.

Trialling an optimised social groups intervention in services to enhance social connectedness and mental health in vulnerable young people (TOGETHER): Study protocol for a feasibility randomised controlled trial

Protocol for a randomised controlled feasibility trial of exercise rehabilitation for people with postural tachycardia syndrome: the PULSE study, Pilot and Feasibility Studies

Exercise Guidelines for Postural Tachycardia Syndrome

PDF) Feasibility study of an optimised person-centred intervention to improve mental health and reduce antipsychotics amongst people with dementia in care homes: study protocol for a randomised controlled trial

PDF) Protocol for a randomised controlled feasibility trial of exercise rehabilitation for people with postural tachycardia syndrome: the PULSE study

PDF) Tailored exercise management (TEMPO) versus usual care for people aged 80 years or older with hip/knee osteoarthritis: study protocol for a feasibility randomised controlled trial

Volume 6, issue 1 Pilot and Feasibility Studies

CONSORT 2010 statement: extension to randomised pilot and feasibility trials
Endurance Exercise Training in Orthostatic Intolerance
Feasibility of a high-PRotein Mediterranean-style diet and resistance Exercise in cardiac Rehabilitation patients with sarcopenic obesity (PRiMER): Study protocol for a randomised control trial

Nikki Holliday - Design Manager - Coventry University
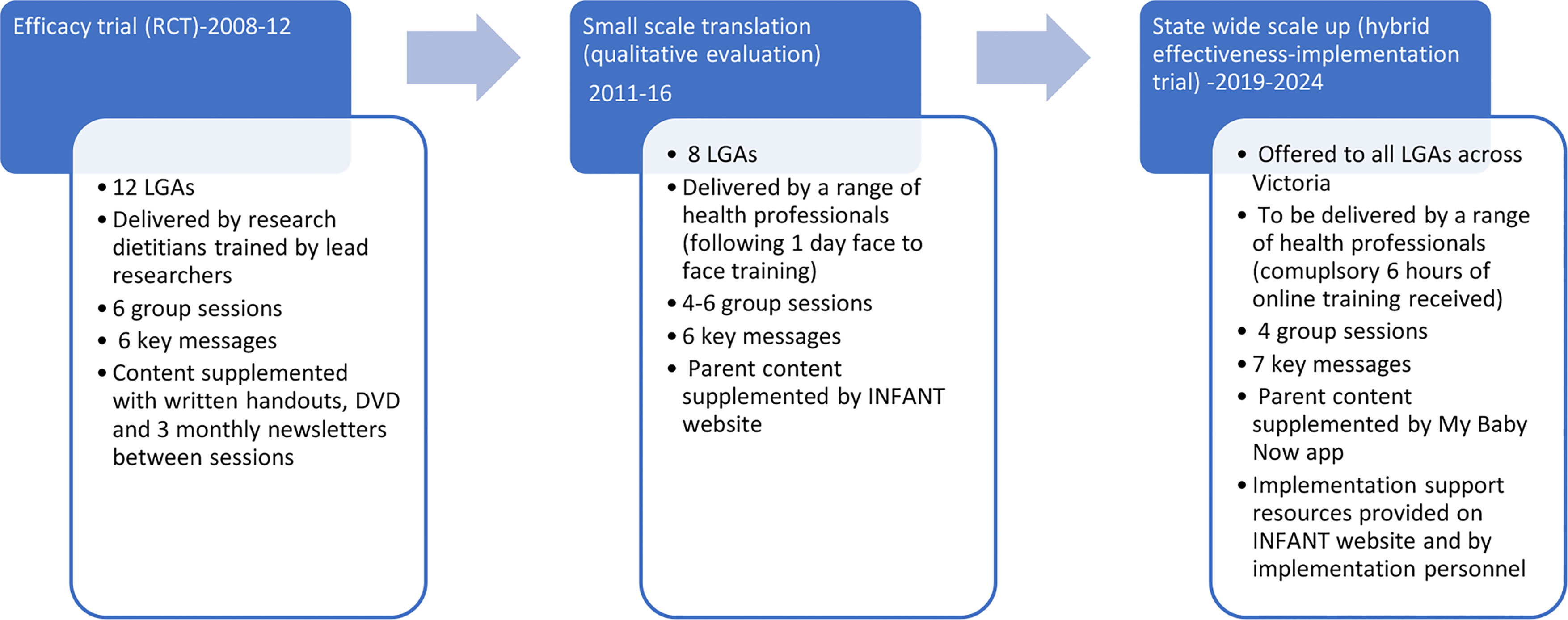
Frontiers Protocol for an Effectiveness-Implementation Hybrid Trial to Evaluate Scale up of an Evidence-Based Intervention Addressing Lifestyle Behaviours From the Start of Life: INFANT

PDF] Endurance Exercise Training in Orthostatic Intolerance: A Randomized, Controlled Trial
Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework

Co-creation of a complex, multicomponent rehabilitation intervention and feasibility trial protocol for the PostUraL tachycardia Syndrome Exercise (PULSE) study, Pilot and Feasibility Studies