
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

At 300K, 36g of glucose present in a litre of its solution has an osmotic pressure of 4.98bar.
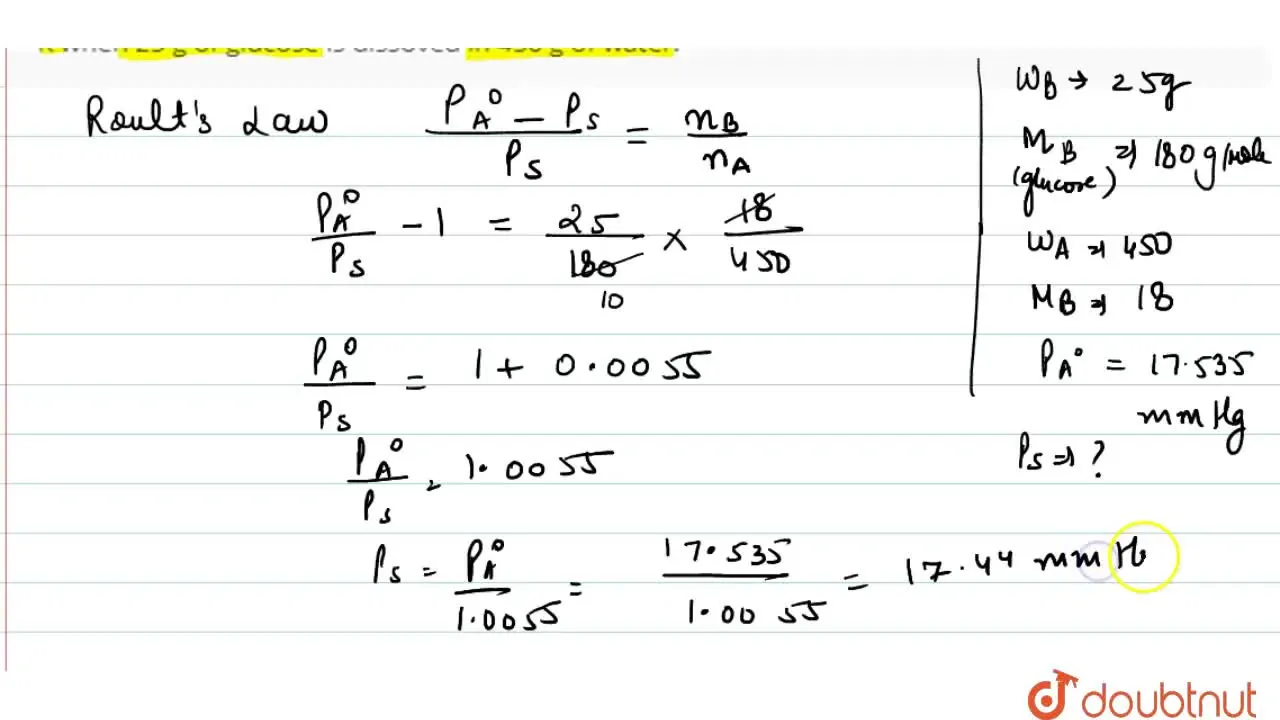
Vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapou

BIL360 DuBois Chapter 5: Transport of Solutes and Water Flashcards

B14. At 300k, 30g of glucose, C6H1206 present per litse en its solutior has an osmotic pressure of 4.98 bar. If the asmotic pressure of another glucose solution is 1.52 bar the

N 40 41 1 hi-res stock photography and images - Page 6 - Alamy

A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 m

At 300 K, 36 gof glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the same

Based on solute - solvent interactions, arrange the following in order

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

Nutrients, Free Full-Text
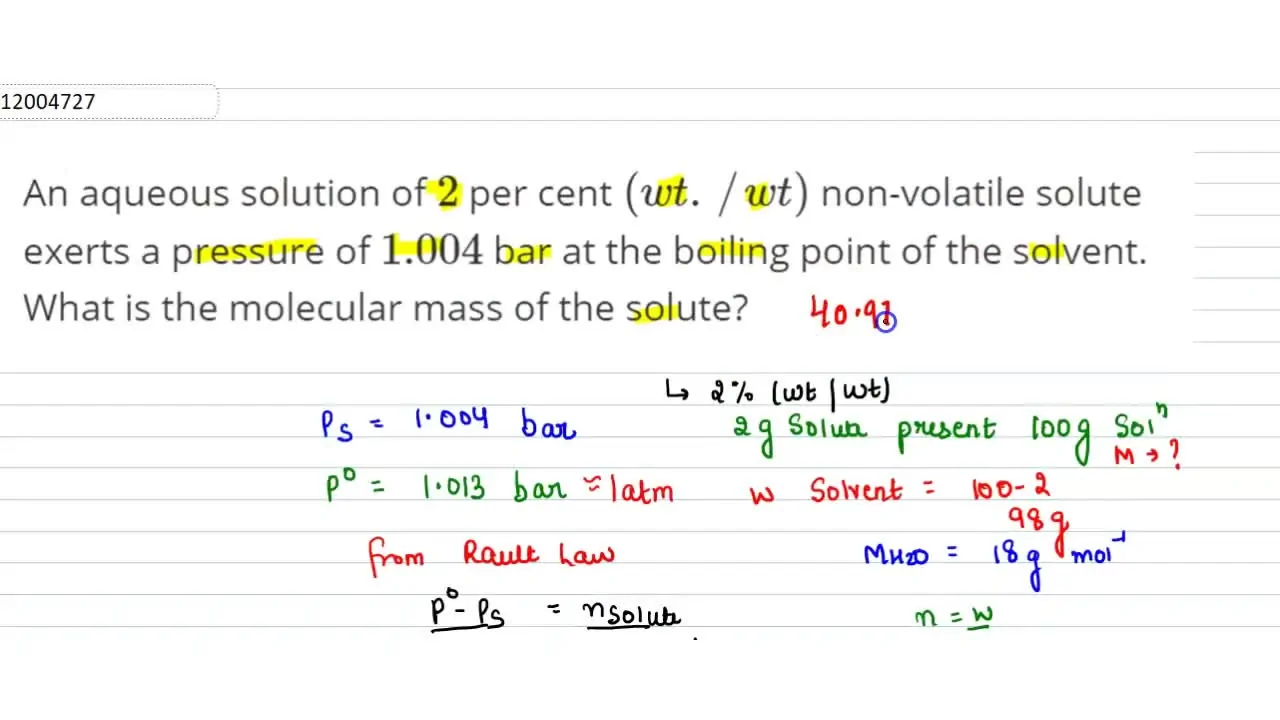
An aqueous solution of 2 per cent (wt.//wt) non-volatile solute exerts










