Description

What is the mass of carbon dioxide which contains the same number of molecules as are contained in 40 g of oxygen?55 g40 g32 g44 g
4.4 gram of carbon dioxide and 2.24 litres of hydrogen molecule at STP are mixed in a container. What will be the total number of molecule present in the container? - Quora

What mass of carbon is present in 44g of carbon dioxide? - Quora

Radiative energy flux variations from 2000 – 2020

How to Find the Mass of One Molecule of Carbon dioxide (CO2)
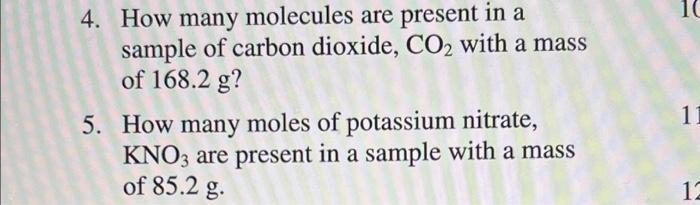
Solved 10 4. How many molecules are present in a sample of
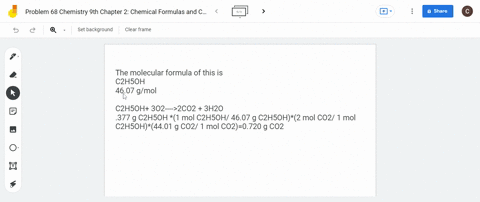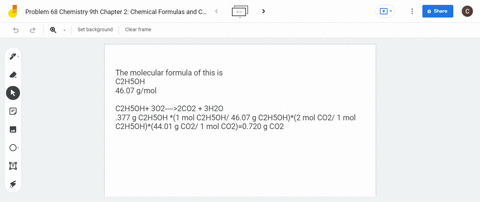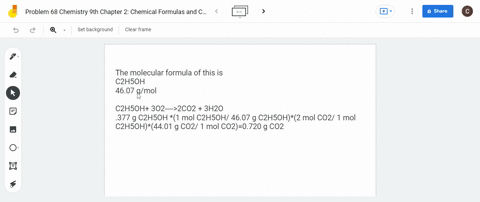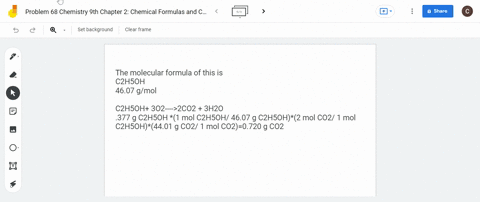
SOLVED: What is the mass of carbon present in 44g of carbon dioxide.
What is the mass of one molecule of CO2? - Quora
What is the volume of oxygen required to burn 36 grams of carbon completely at STP? - Quora
Related products
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95

Cadbury Crunchie Bar Chocolate Bar from Canada – Candy Funhouse US

Patatine Lay's Classica 20 bustine x 44 g Lay's
BB Cream Spf 44 Clareador Escuro N30 Latika 30g - PanVel Farmácias
$ 27.00USD
Score 4.9(283)
In stock
Continue to book
$ 27.00USD
Score 4.9(283)
In stock
Continue to book
©2018-2024, followfire.info, Inc. or its affiliates



