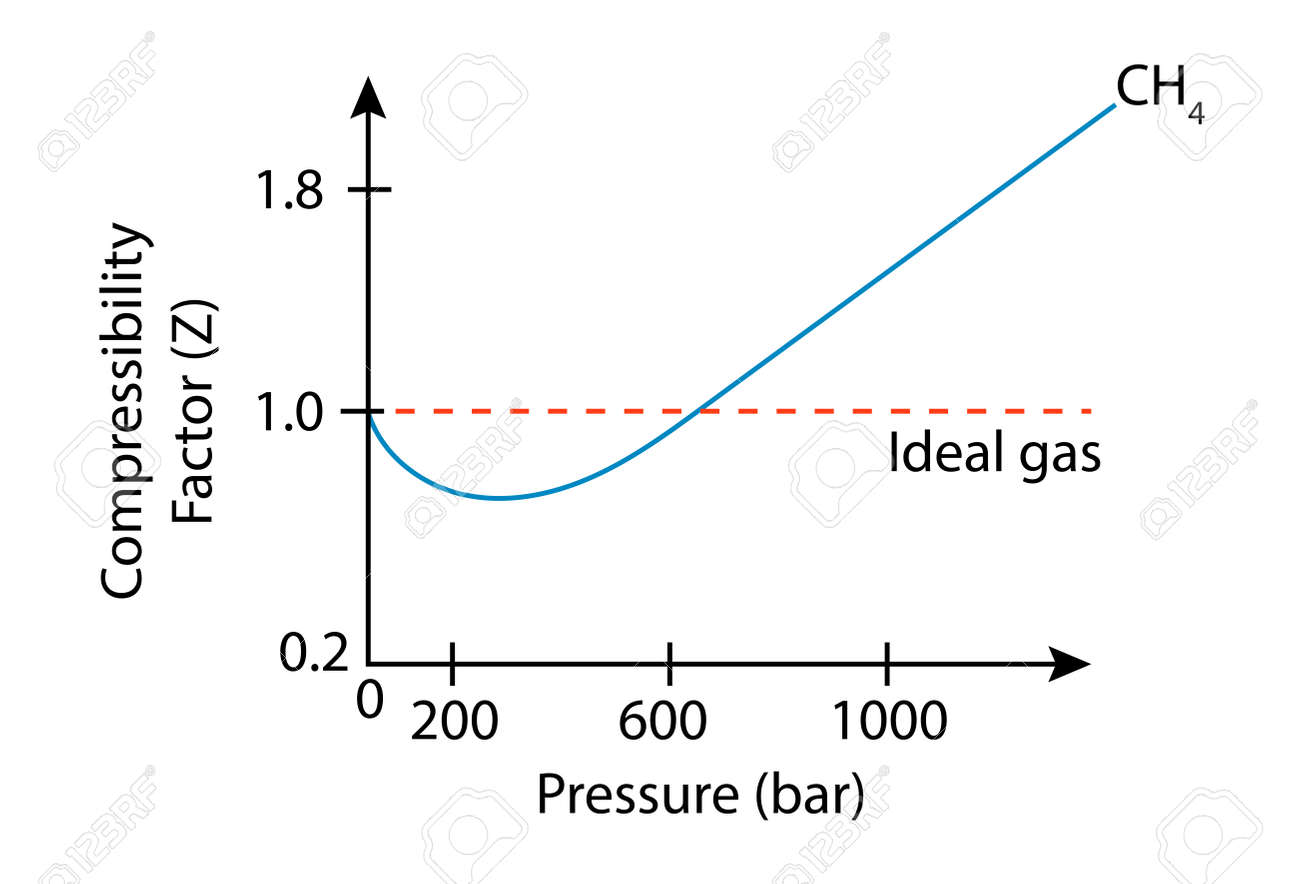
Compressibility factor Z = PV / nRT is plotted against pressure as

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
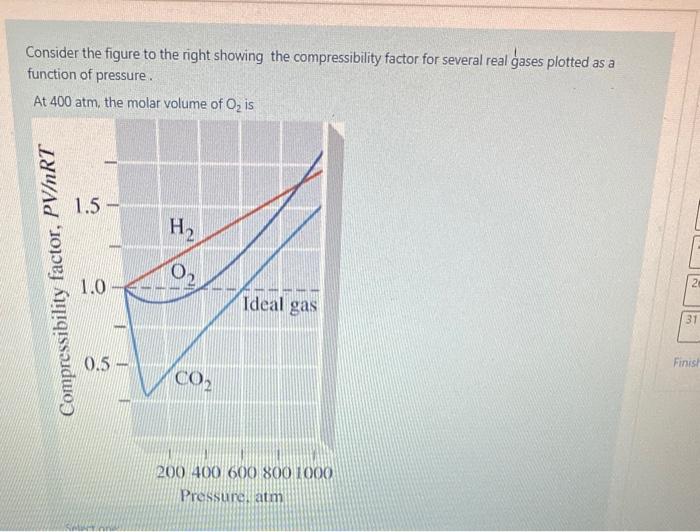
Solved Consider the figure to the right showing the
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following
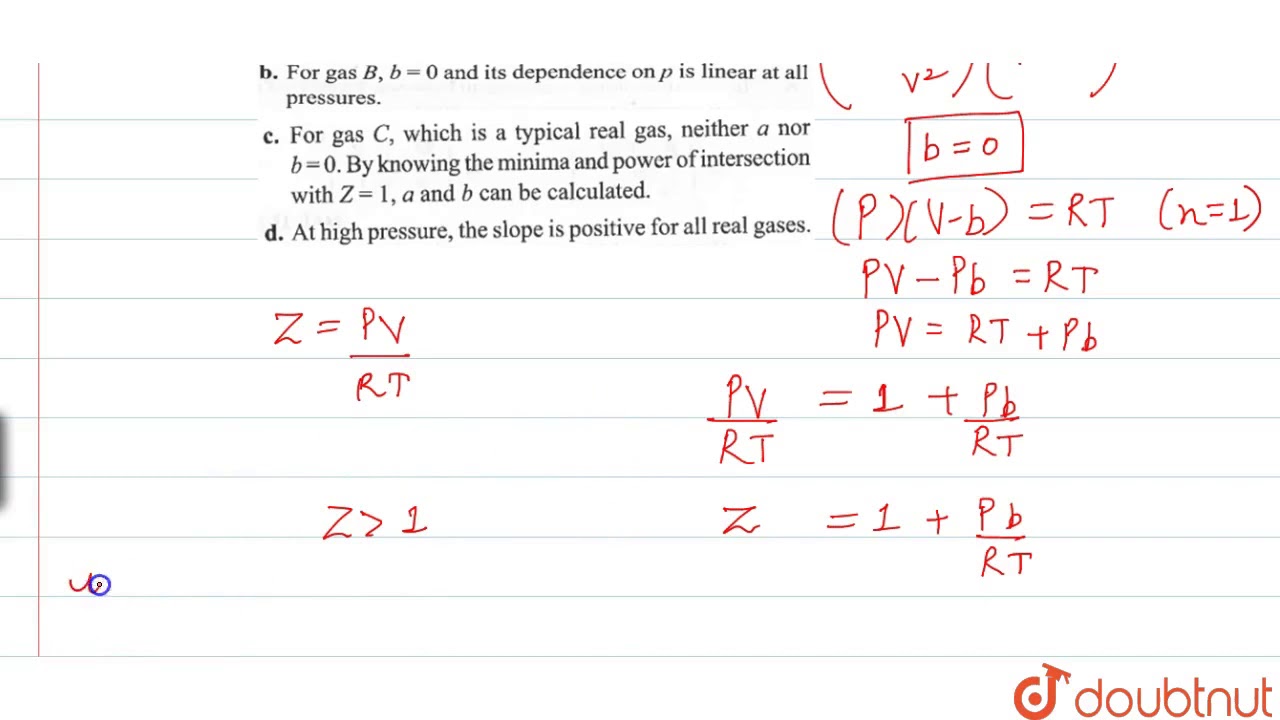
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees
Why the graph of a pressure against volume of a fixed amount of a gas a curve and not a straight line? - Quora

Non-Ideal Gas Behavior Chemistry: Atoms First
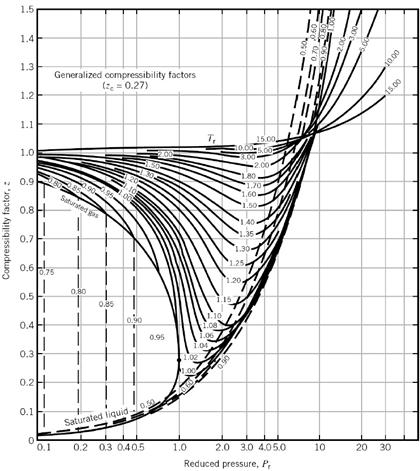
Z= PVm / RT for gases as a function of the reduced
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange