
SOLVED: Diatomic ideal gas (γ = 1.40) confined to a cylinder through a closed cycle. Initially, the gas is at Pi, Vir, and Ti. First, its pressure doubles under constant volume. It

m.media-/images/I/71VgHcCCUAL._AC_UF894

⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…
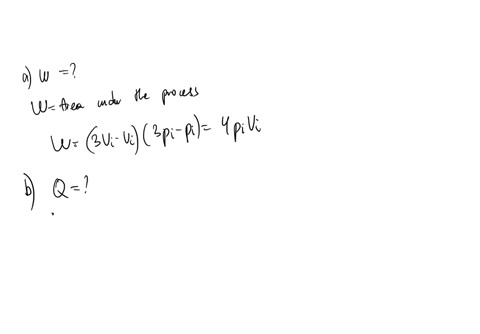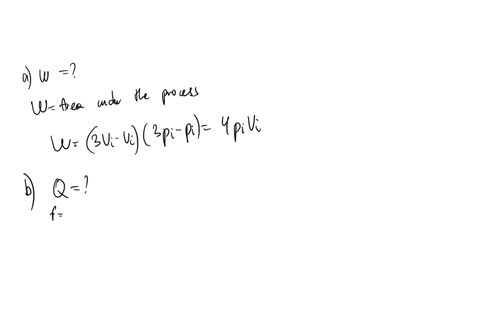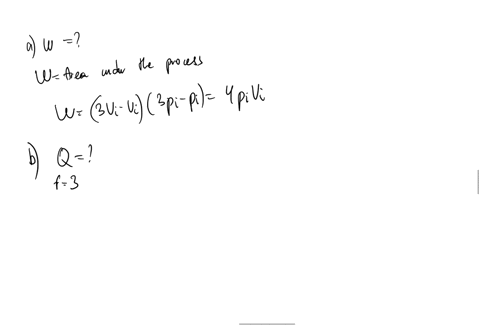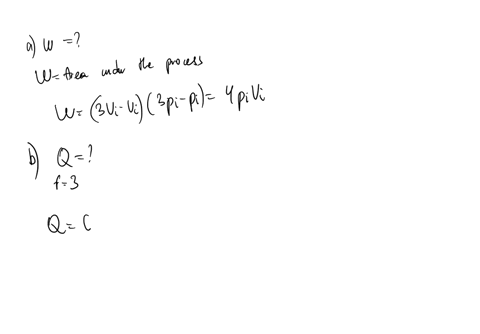
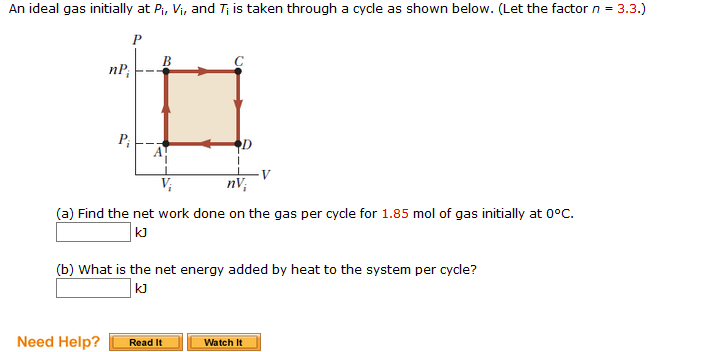
SOLVED: An ideal gas initially at Pi' Vi' and Ti is taken through a cycle as shown below. (Let the factor n 2.8.) nP; nV; a) Find the net work done on

1 mole of an ideal gas at initial temperature of T K does 6 R joules of work adiabatically. If the ratio of specific heats of this gas at constant pressure and
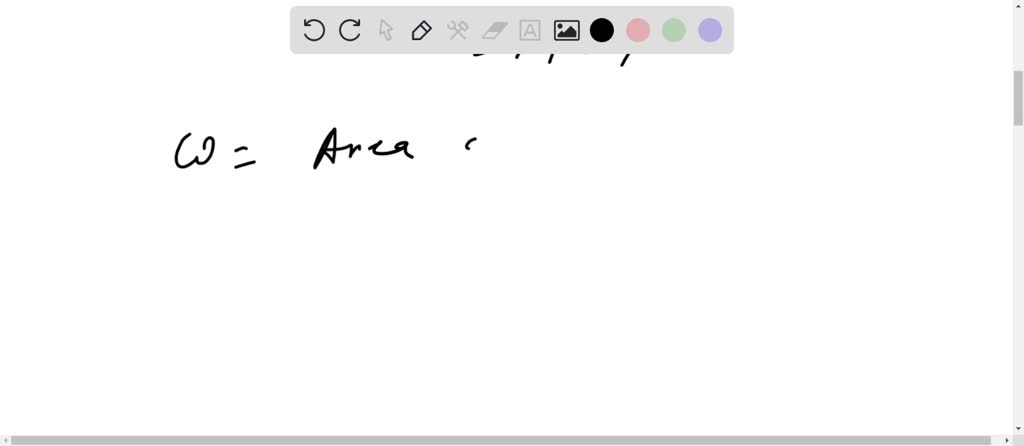
Solved An ideal gas initially at P_i, V_i, and T_i is taken
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n 3.6.) nP; P; nV; (a Find the net work done

⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

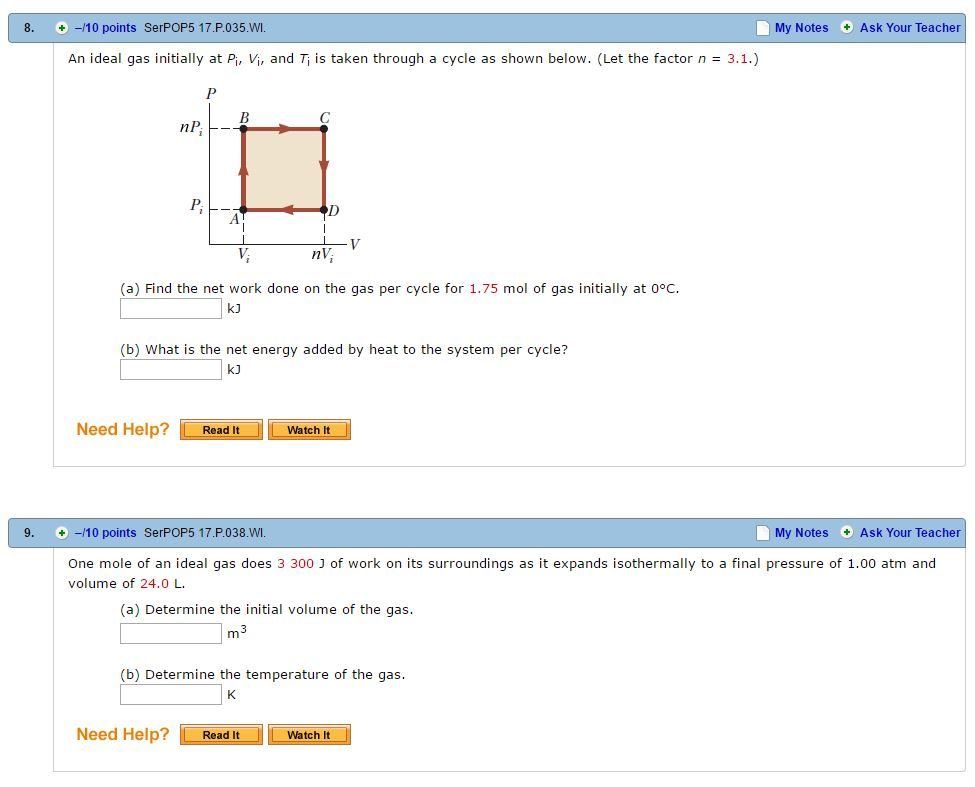
An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
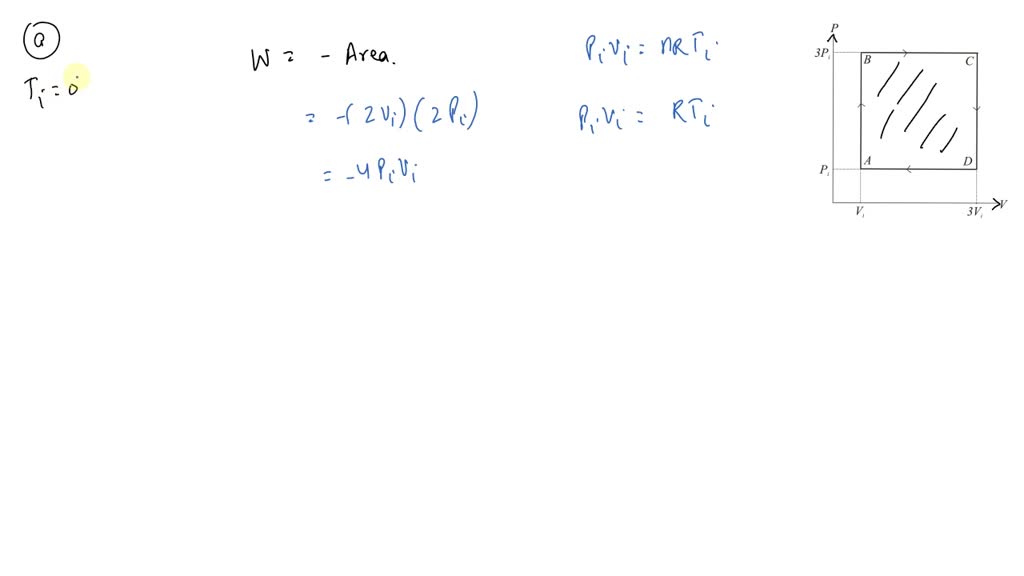
⏩SOLVED:An ideal gas initially at Pi, Vi and Ti is taken through a…