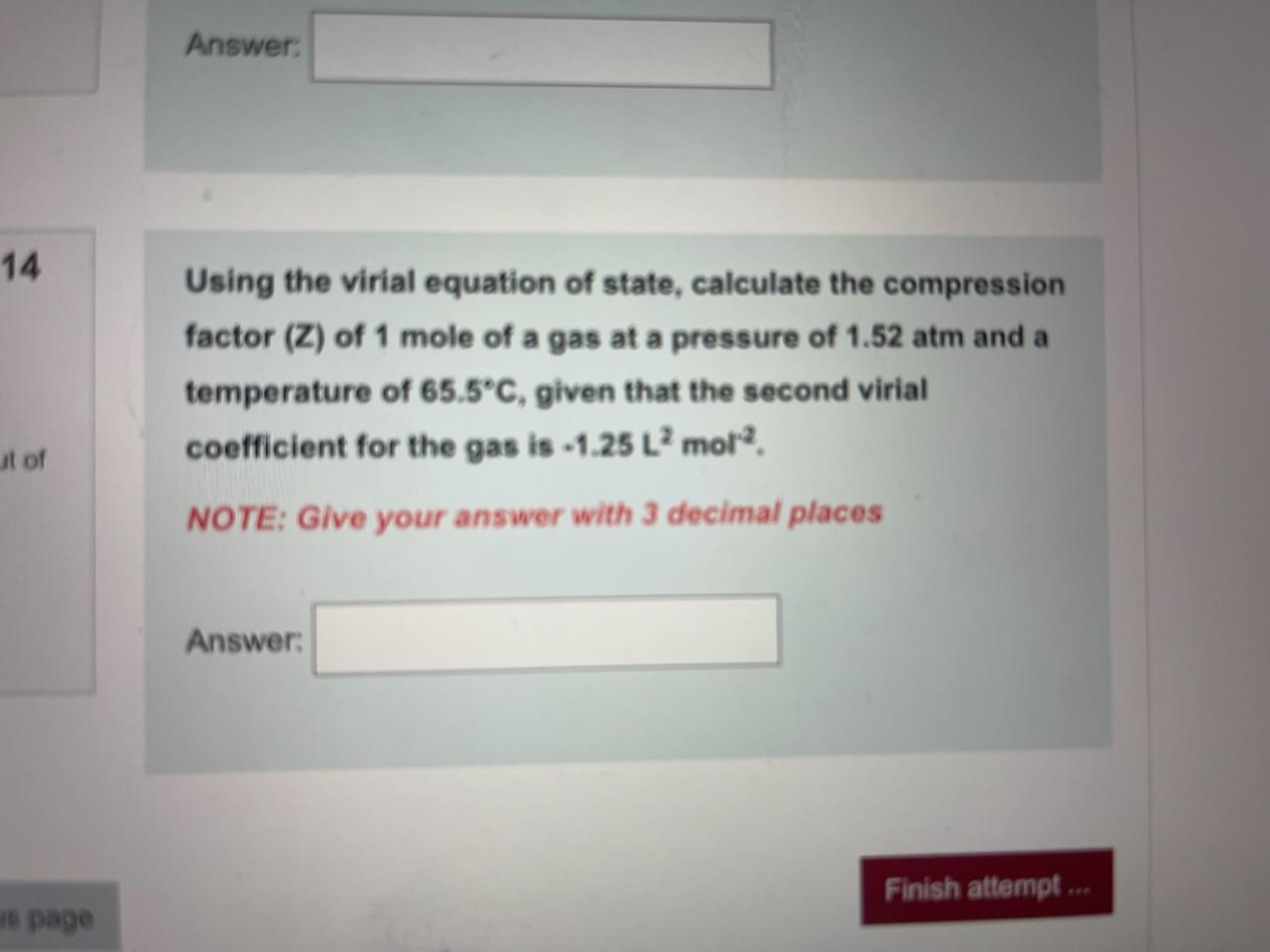
At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{

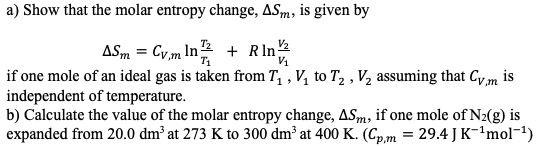
Answered: a) Show that the molar entropy change,…

Random, PDF, Gases

Converting Between Moles and Liters of a Gas at STP

Enabling hydrate-based methane storage under mild operating conditions by periodic mesoporous organosilica nanotubes - ScienceDirect

Chemistry - Unit 3 - Joseph Flashcards

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

Minerals, Free Full-Text

Left) Argon adsorption ( fi lled symbols) and desorption (open

Molarity & Stoichiometry, Definition, Formula & Calculation - Lesson

One mole of argon is expanded according to process equation PV^(1.5)=c
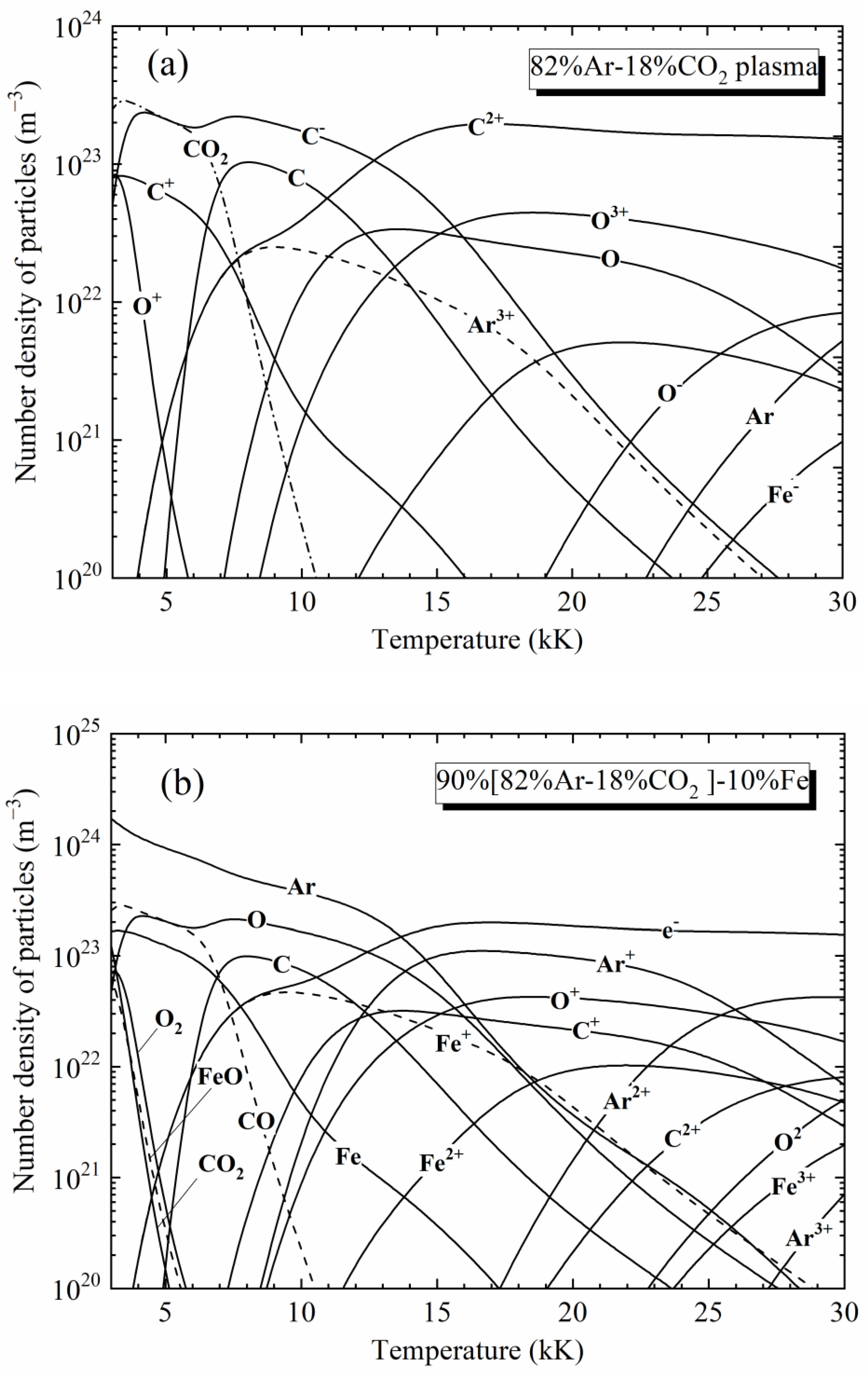
Materials, Free Full-Text
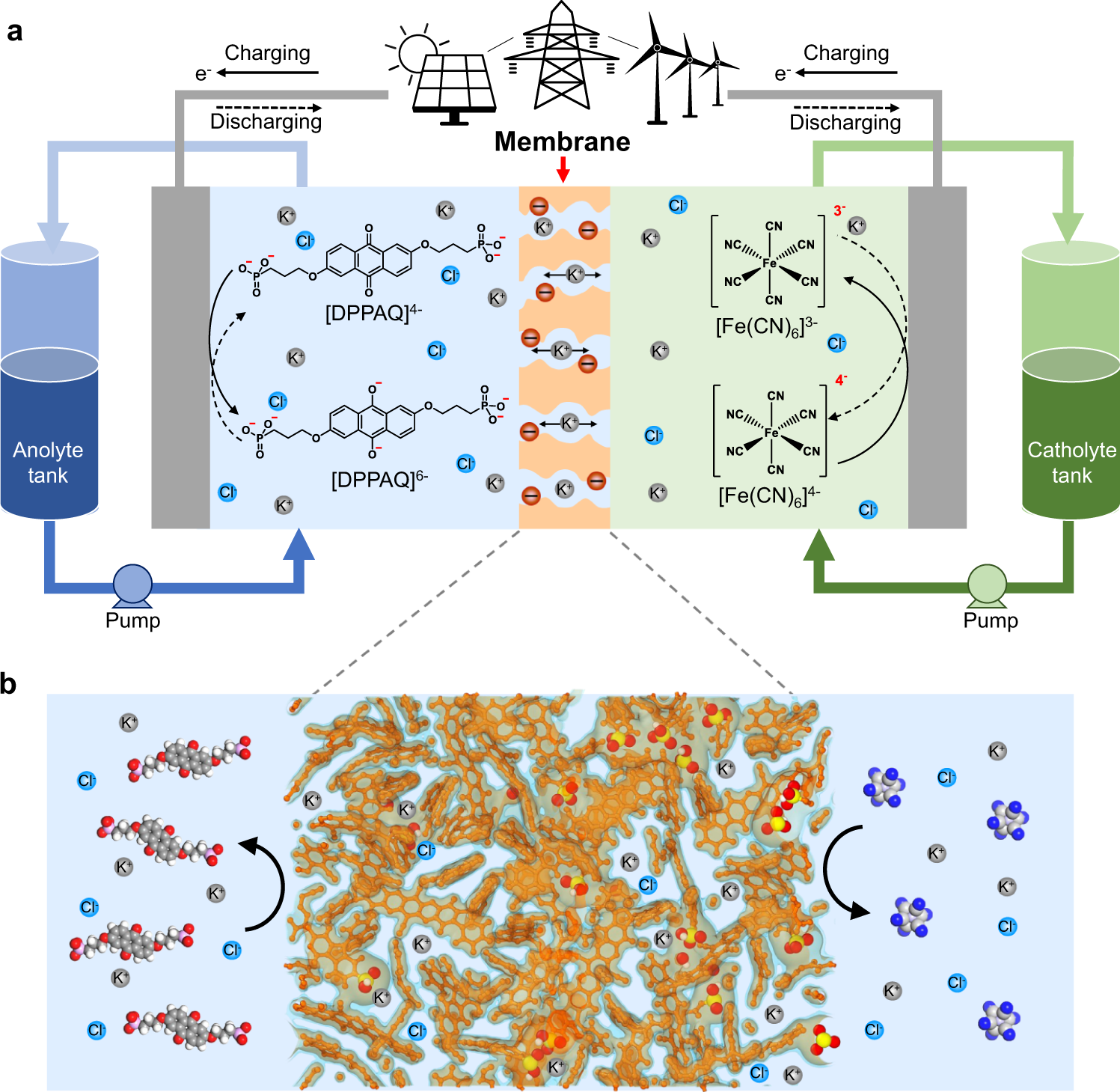
Development of efficient aqueous organic redox flow batteries using ion-sieving sulfonated polymer membranes
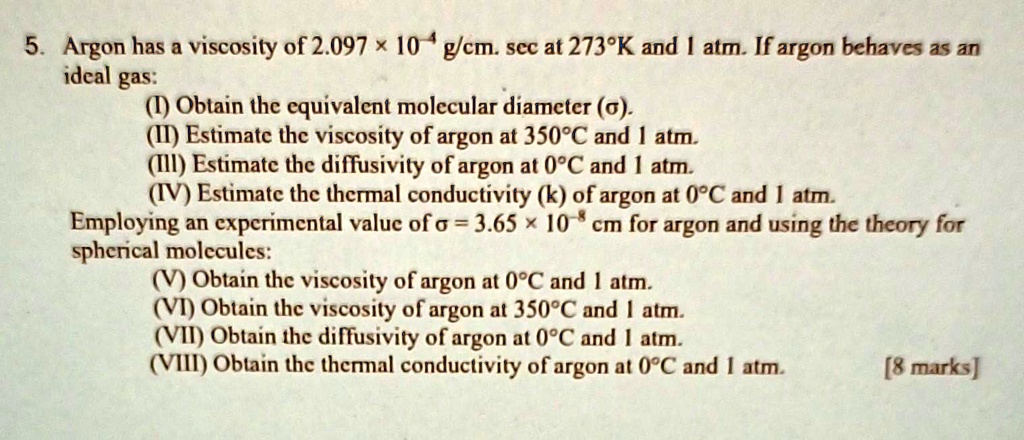
SOLVED: Argon has a viscosity of 2.097 x 10^(-4) g/cm.sec at 273K and 1 atm. If argon behaves as an ideal gas, obtain the equivalent molecular diameter. Estimate the viscosity of argon

CO 2 sorption isotherm of 1 carried out at 273 K, closed circles

Gas Laws