20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

Solved The ideal gas law can represent the
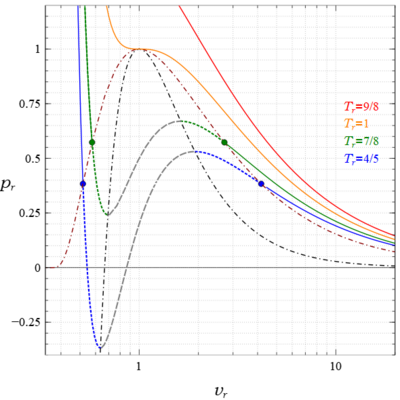
Van der Waals equation - Wikipedia

If Z is a compressibility factor, Van der Waals equation at low pressure can be written as

Solved APPENDIX Problem 1: Molar Volume and Compressibility
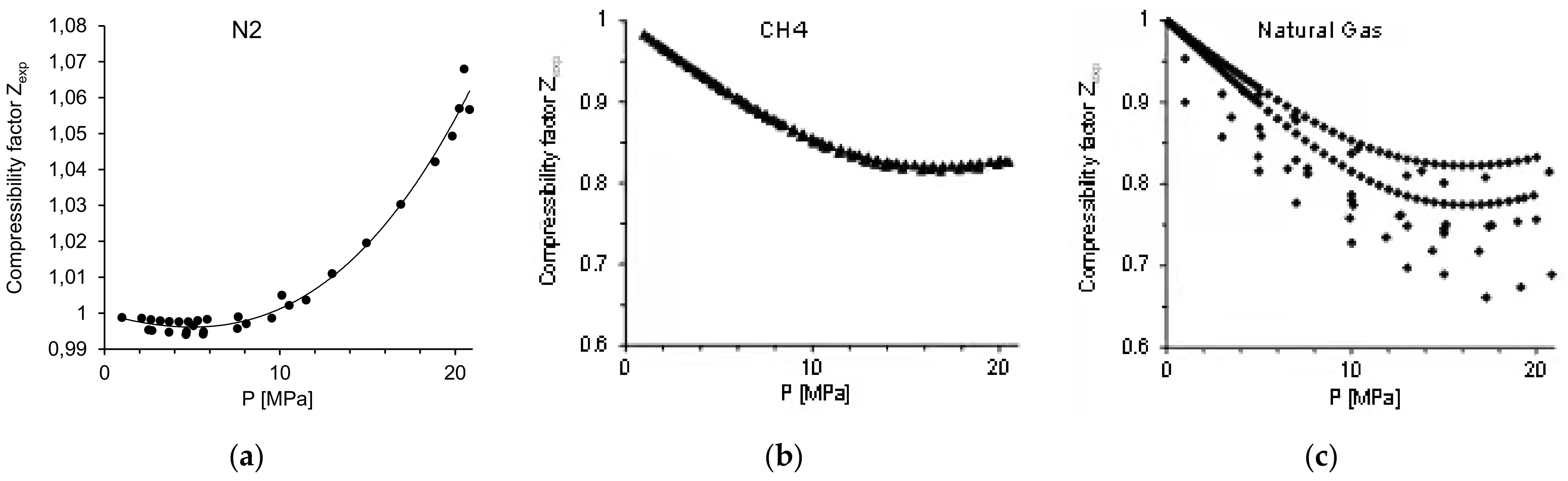
Metals, Free Full-Text

Compressibility factor - Wikipedia
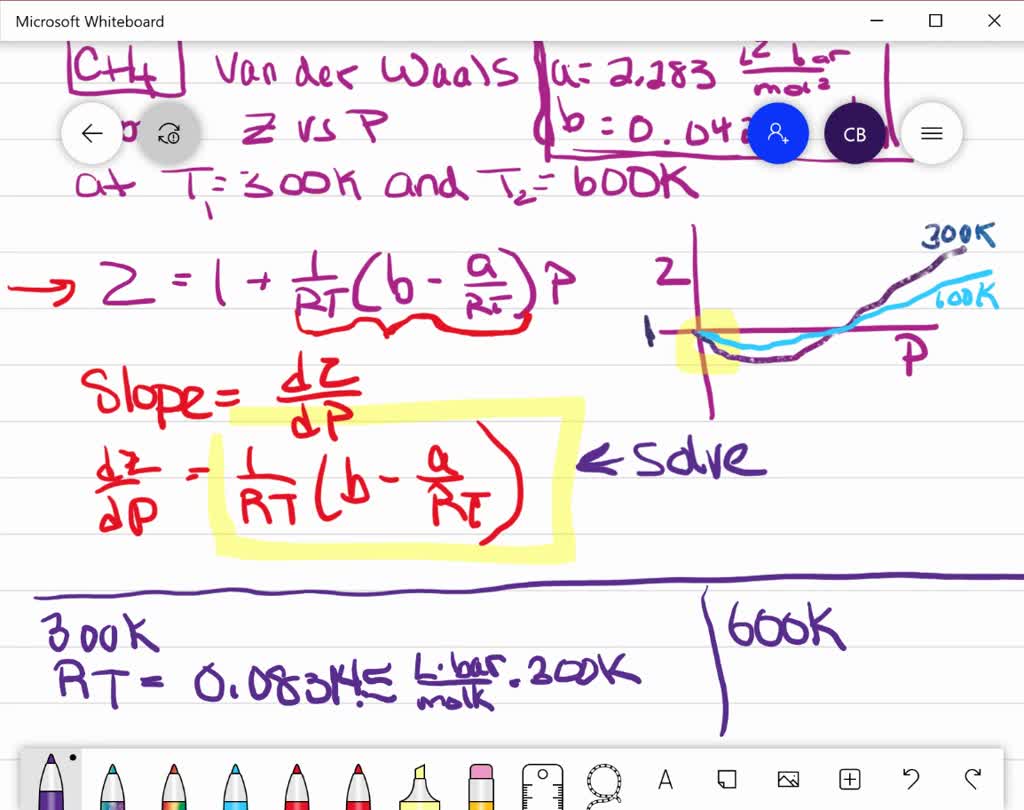
⏩SOLVED:Use the van der Waals constants for CH4 in Table 1.3 to…

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download

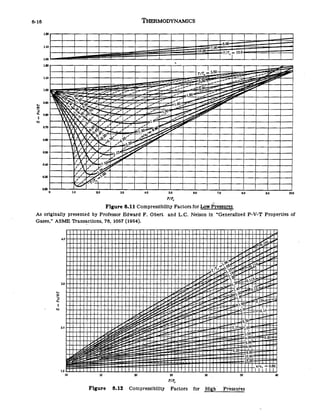
Objectives_template

If Z is a compressibility factor, van der Waals equation at low pressure ..

If Z is compressibility factor, vander Waals equation low pressure can be written as