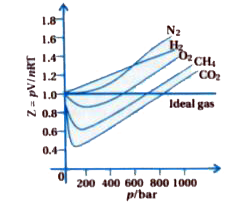
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state
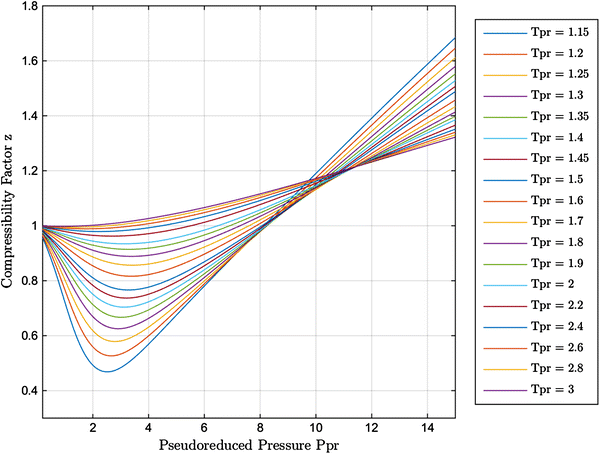
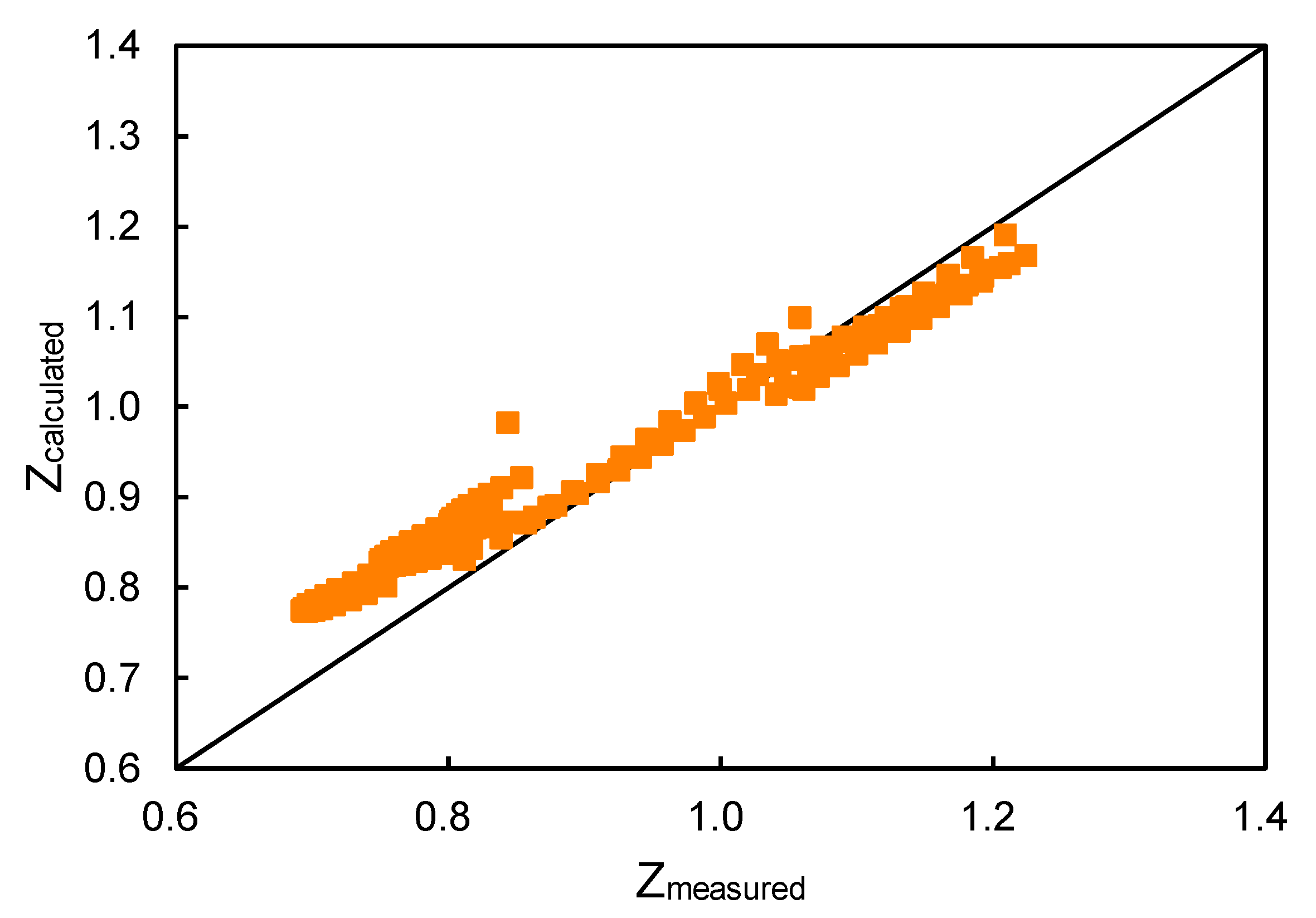
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

Compressibility factor (gases) - Citizendium


Carbon dioxide compressibility factor determination using a robust

WPILARIVIANN ZU 60. ollowing compressibility factor (2) vs pressure graph 300 K, the compresability of Cheatre 200 bar deviates from ideal behaviour because Compressibility Factor (2) Ideal gas 02 0 200 600

Compressibility factor (gases) - Knowino

KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
Gujrati] Explain compressibility factor (Z).
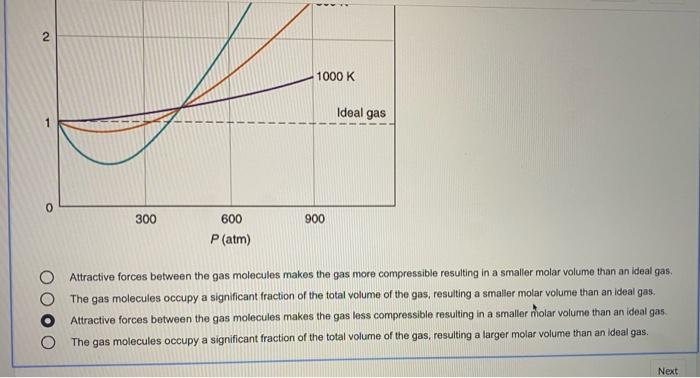
Solved 6 1 point The plot below shows how compressibility

Compressibility Factor Calculator

physical chemistry - Compressibility Factor Graph - Which gas

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1