At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to
Why is the term that corrects for volume, negative and the term

physical chemistry - Why do some gases have lower value of Z for a

At low pressure, Van der Waal's equation is reduced to [P+dfrac{a
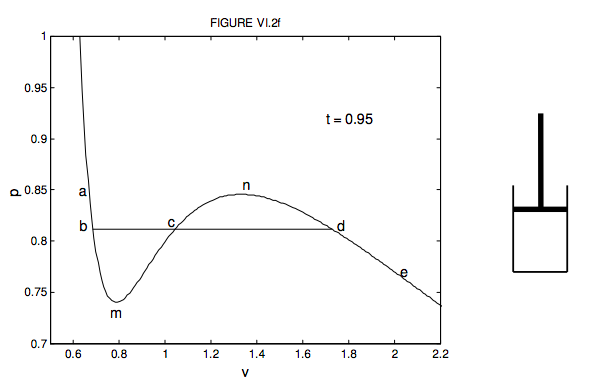
6.3: Van der Waals and Other Gases - Physics LibreTexts

Compressibility factor (Z) a real gas moderately low pressure is

2. U 0.52, 0.68, 0.74 At low pressure, the comprensibility factor
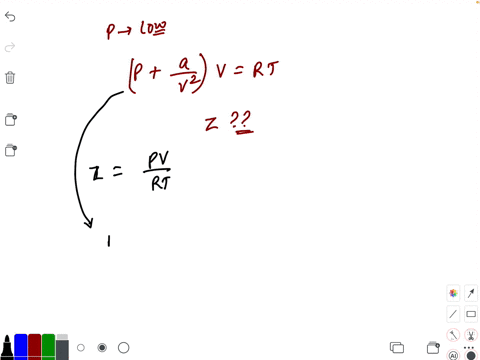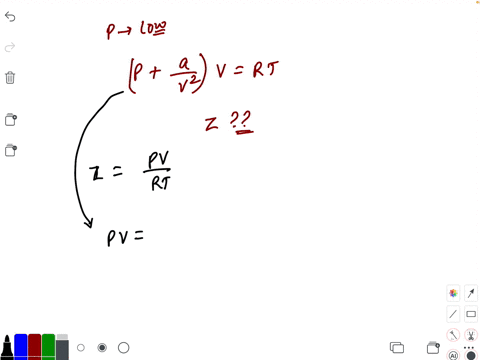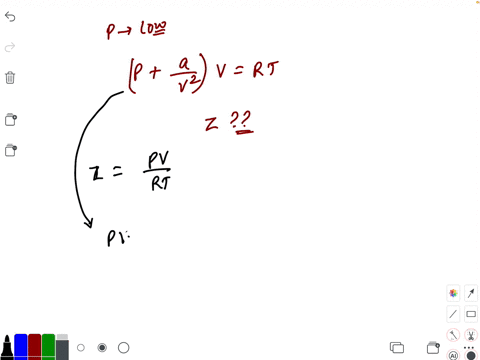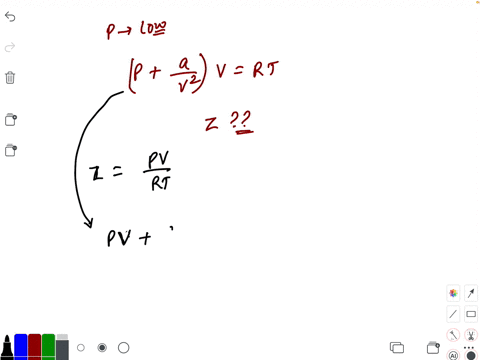
⏩SOLVED:If Z is a compressibility factor, van der Waals equation
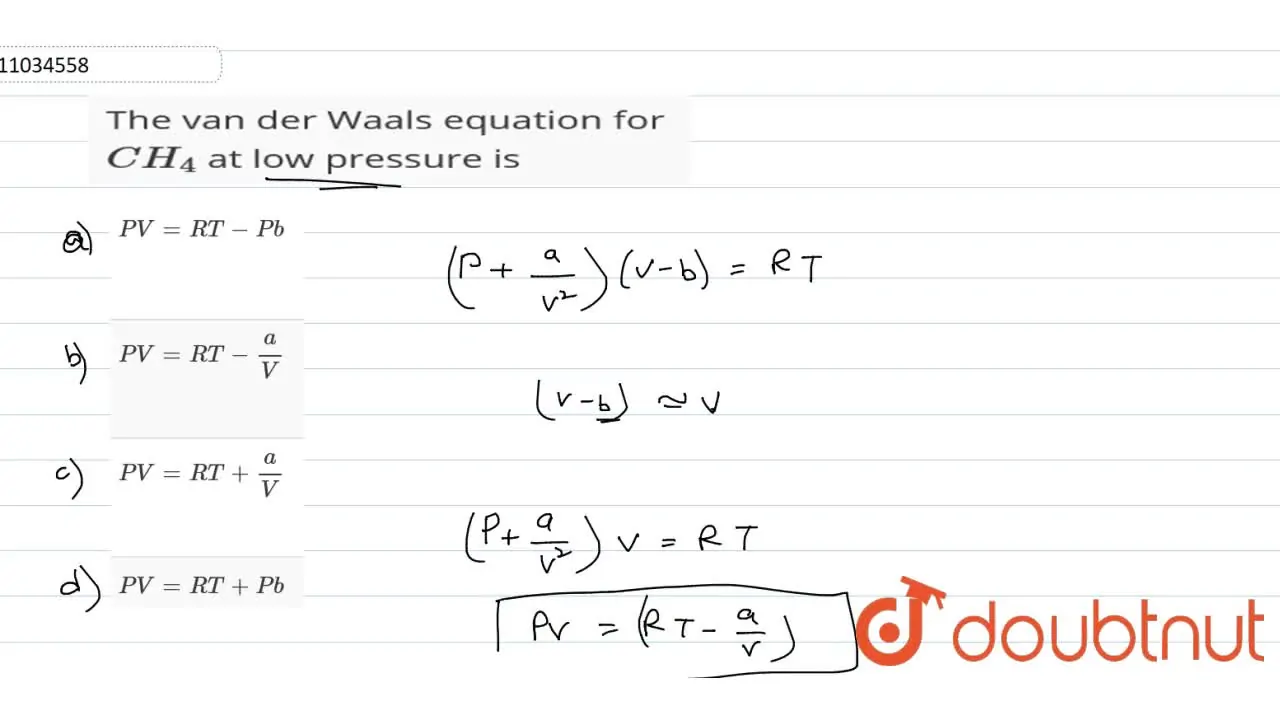
The van der Waals equation for CH(4) at low pressure is

At low pressures (for 1 mole), the van der Waal's equation is

SOLVED: I need the answer as soon as possible. 16. If Z is a
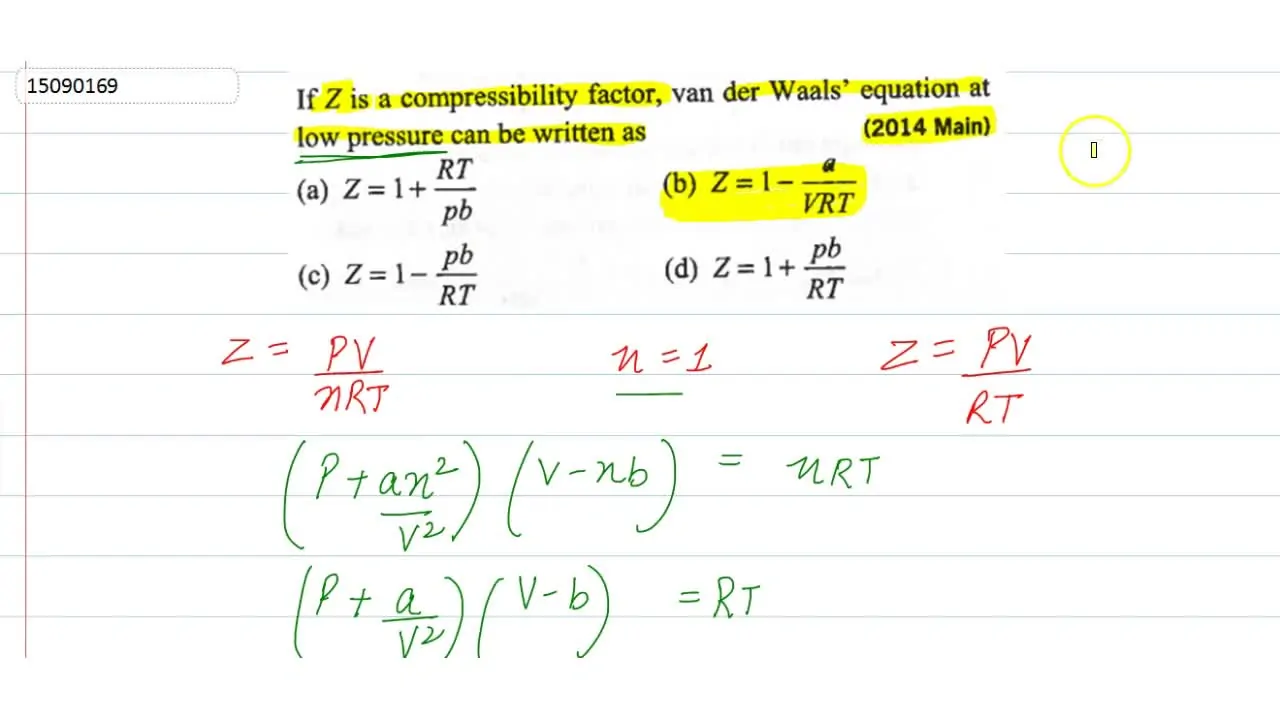
If Z is a compressibility factor, van der Waals' equation at low press

Problem Set 2 Solutions