At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

The van der waals equation for a gas is (P + a/v2)(V-b) = RT where

Van der Waals equation: van der Walls EOS, [Pr*3/Vr^2] [3Vr-1] =
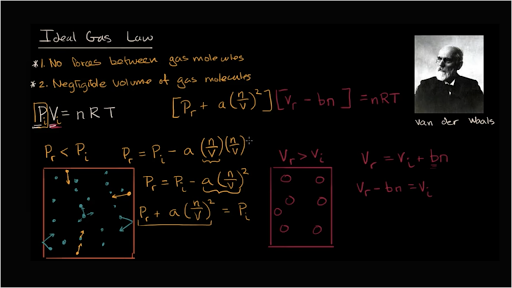
The van der Waals equation (video)
At relatively high pressures, the van der Waal's equation of st

At low pressures (for 1 mole), the van der Waal's equation is
Solving Maxwell Relations Homework with Van der Waals Gas

At high temperature and low pressure, the van der Waals' equation

Example 5. According to van der Waals' equation pressure (P

At extremely low pressure the Van der Waals equation of one mole

Van Der Waals Equation - an overview

Bengali] At a low pressure, the van der waals equation reduces to (P+

Van der Waals Equation of State in Python
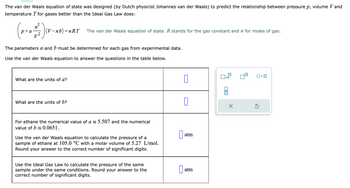
Answered: The van der Waals equation of state was…







