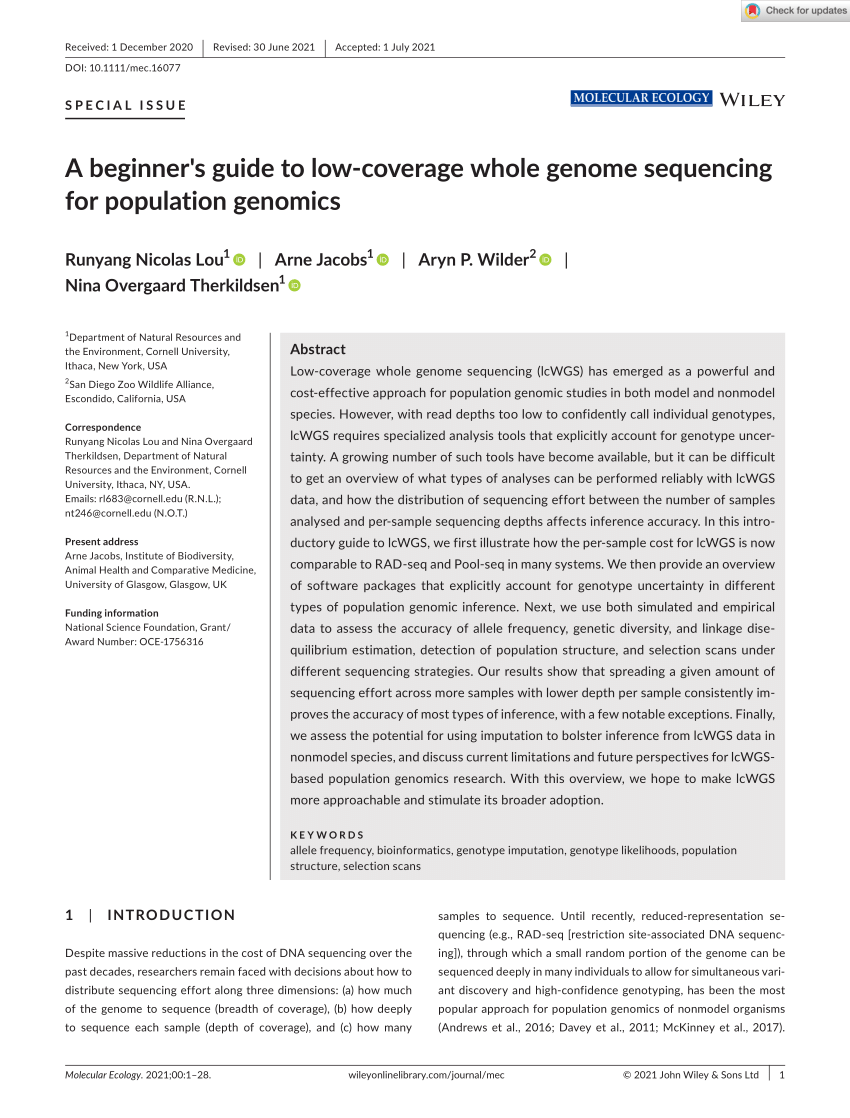
Evaluating coverage bias in next-generation sequencing of Escherichia coli
Whole-genome sequencing is essential to many facets of infectious disease research. However, technical limitations such as bias in coverage and tagmentation, and difficulties characterising genomic regions with extreme GC content have created significant obstacles in its use. Illumina has claimed that the recently released DNA Prep library preparation kit, formerly known as Nextera Flex, overcomes some of these limitations. This study aimed to assess bias in coverage, tagmentation, GC content, average fragment size distribution, and de novo assembly quality using both the Nextera XT and DNA Prep kits from Illumina. When performing whole-genome sequencing on Escherichia coli and where coverage bias is the main concern, the DNA Prep kit may provide higher quality results; though de novo assembly quality, tagmentation bias and GC content related bias are unlikely to improve. Based on these results, laboratories with existing workflows based on Nextera XT would see minor benefits in transitioning to the DNA Prep kit if they were primarily studying organisms with neutral GC content.
Publications — Greenleaf Lab @ Stanford

Phables: from fragmented assemblies to high-quality bacteriophage genomes

AMR-Diag: Neural network based genotype-to-phenotype prediction of resistance towards β-lactams in Escherichia coli and Klebsiella pneumoniae - Computational and Structural Biotechnology Journal

Measuring sequencer size bias using REcount: a novel method for highly accurate Illumina sequencing-based quantification, Genome Biology

A simplified whole-genome sequencing library preparation workflow
Evaluating coverage bias in next-generation sequencing of Escherichia coli
Evaluating coverage bias in next-generation sequencing of Escherichia coli
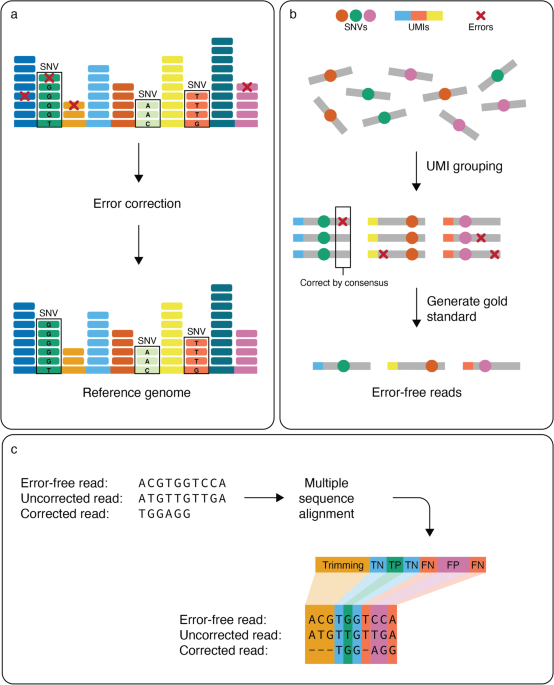
Benchmarking of computational error-correction methods for next-generation sequencing data, Genome Biology

On-and off-target editing analysis in extraction-free libraries. (A)

PDF] Summarizing and correcting the GC content bias in high-throughput sequencing

Evaluation of the sequencing depth of coverage across the three library

Comparison of different nucleosome dissociation methods in

PDF) The efficiency of Nextera XT tagmentation depends on G and C bases in the binding motif leading to uneven coverage in bacterial species with low and neutral GC-content
Development of a versatile high-throughput mutagenesis assay with multiplexed short-read NGS using DNA-barcoded supF shuttle vector library amplified in E. coli