For H(2) gas, the compressibility factor,Z = PV //n RT is

For H(2) gas, the compressibility factor,Z = PV //n RT is

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i) What is the value of Z for an

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
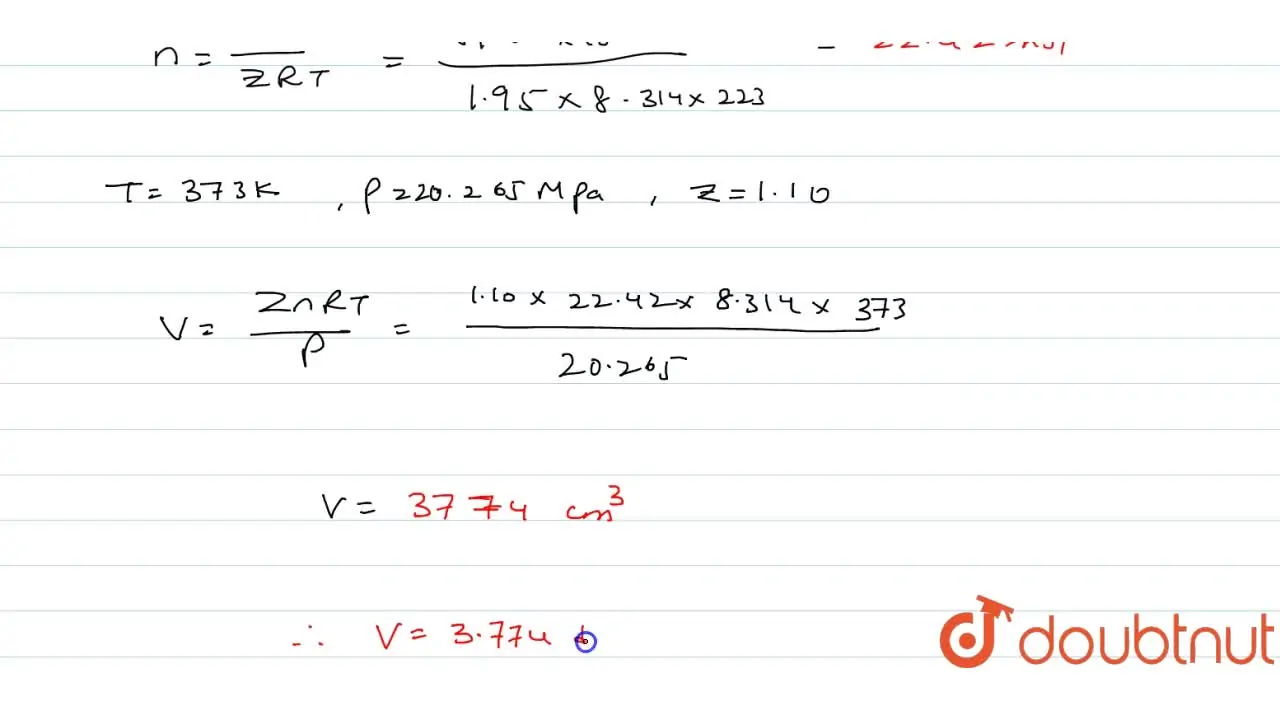
The compressibility factor (Z=PV//nRT) for N(2) at 223 K and 81.06 MPa

Which of the following statements is/are correct? (a) all real gases are less compressible

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

Why H2 gas does not show the Joule-Thomson effect?

The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement