OneClass: For a real gas, the compressibility factor, Z, is


Gas Compressibility - an overview

حرارة وديناميكا حرارية - ppt download

ideal gas - Compressibility factor and deviation from ideality - Chemistry Stack Exchange
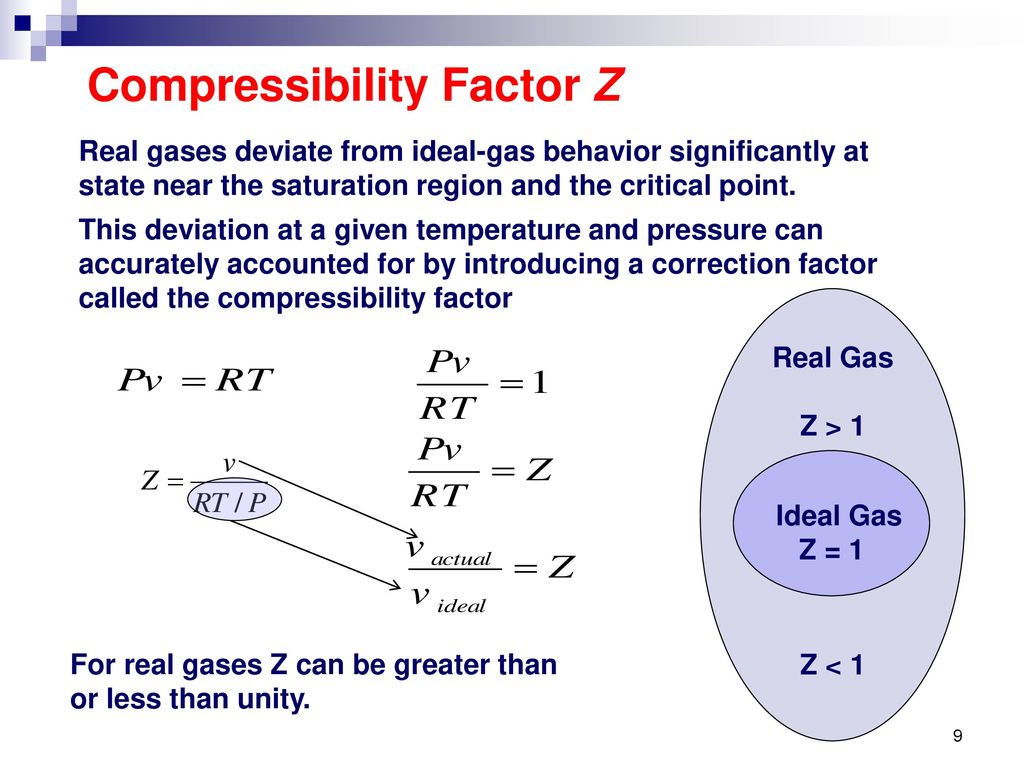
The Ideal Gas. - ppt download
Applied Sciences, Free Full-Text

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

Compressibility factor (z): real gases deviate from ideal behav-Turito

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
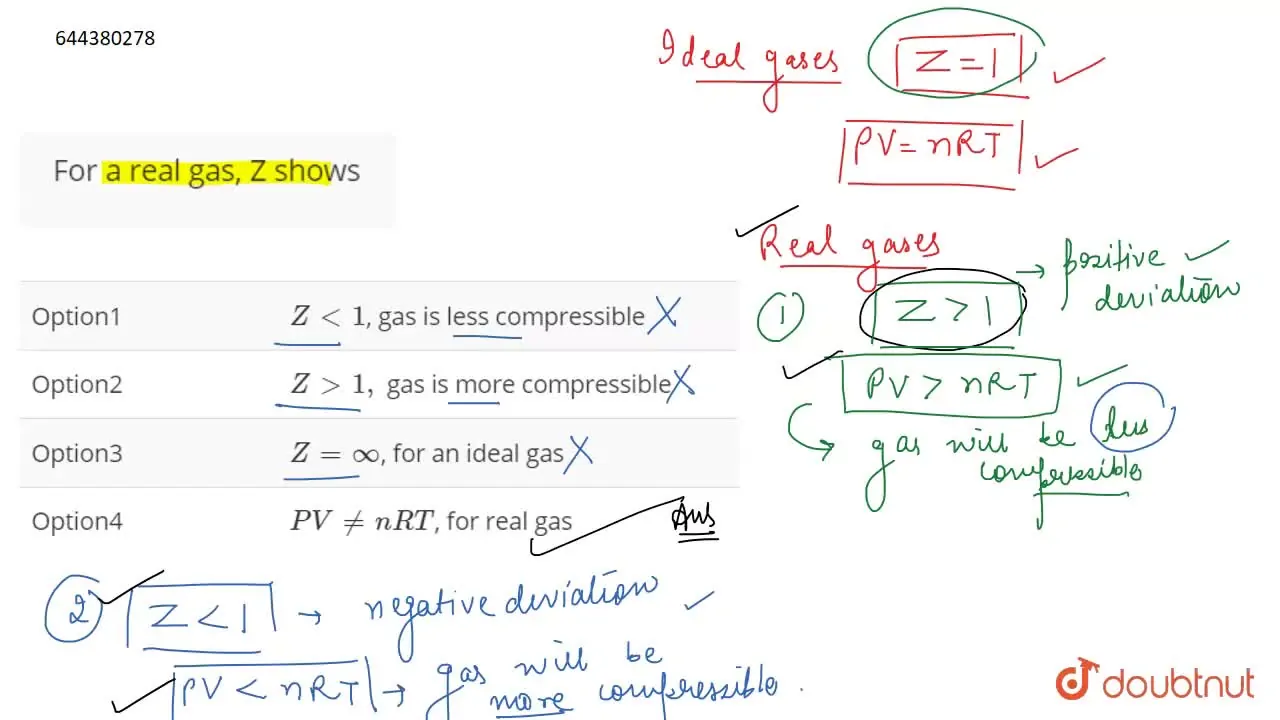
For a real gas, Z shows

OneClass: Estimate the specific volume of helium at -254 degree C and 287 kPa by: (a) Ideal Gas Law (

States of Matter Class 11 Notes CBSE Chemistry Chapter 5 [PDF]

Non-Ideal Gas Behavior Chemistry: Atoms First

For an ideal gas, the value of compressibility factor `Z(=(pVm)/(RT))` is

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!






.png)