The compressibility factor Z for an ideal gas will be

The compressibility factor Z for an ideal gas will be

Compressibility factor - Wikipedia

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
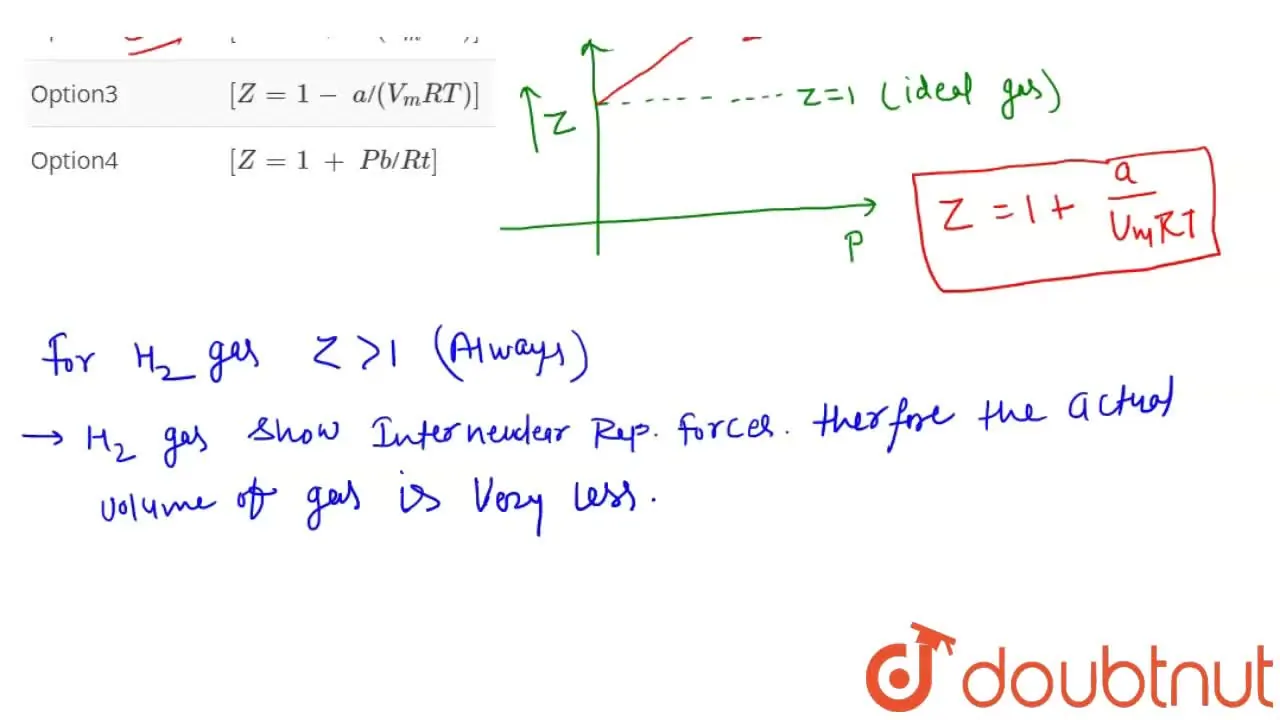
Compressibility factor (Z) for H2 (g) at STP is

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Class Notes on Compressibility of a Real Gas, CH 417, Study notes Physical Chemistry
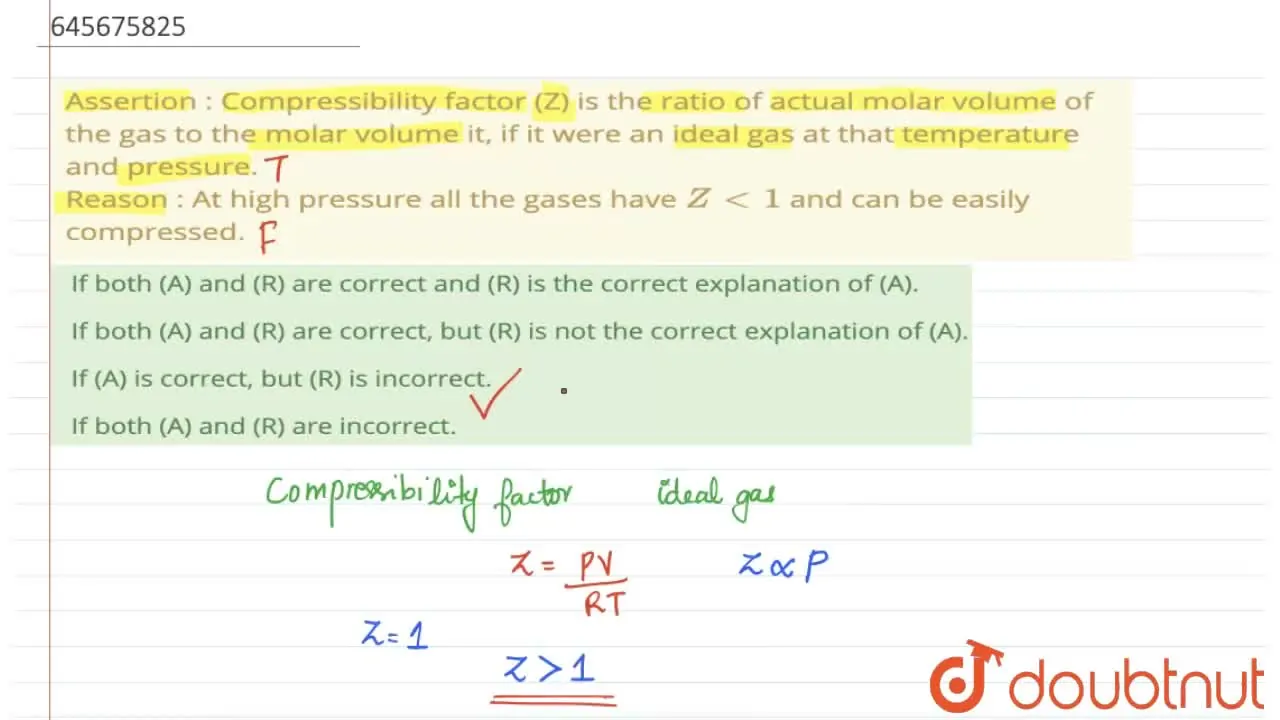
Malayalam] If (A) is correct, but (R) is incorrect.
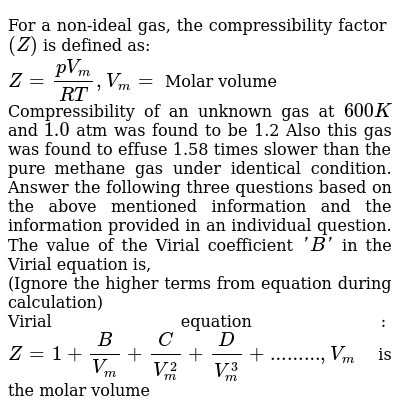
For a non-ideal gas, the compressibility factor (Z) is defined as: Z
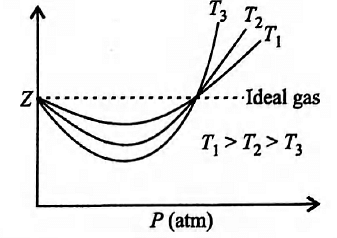
Boyle?? temperature or Boyle point is the temperat
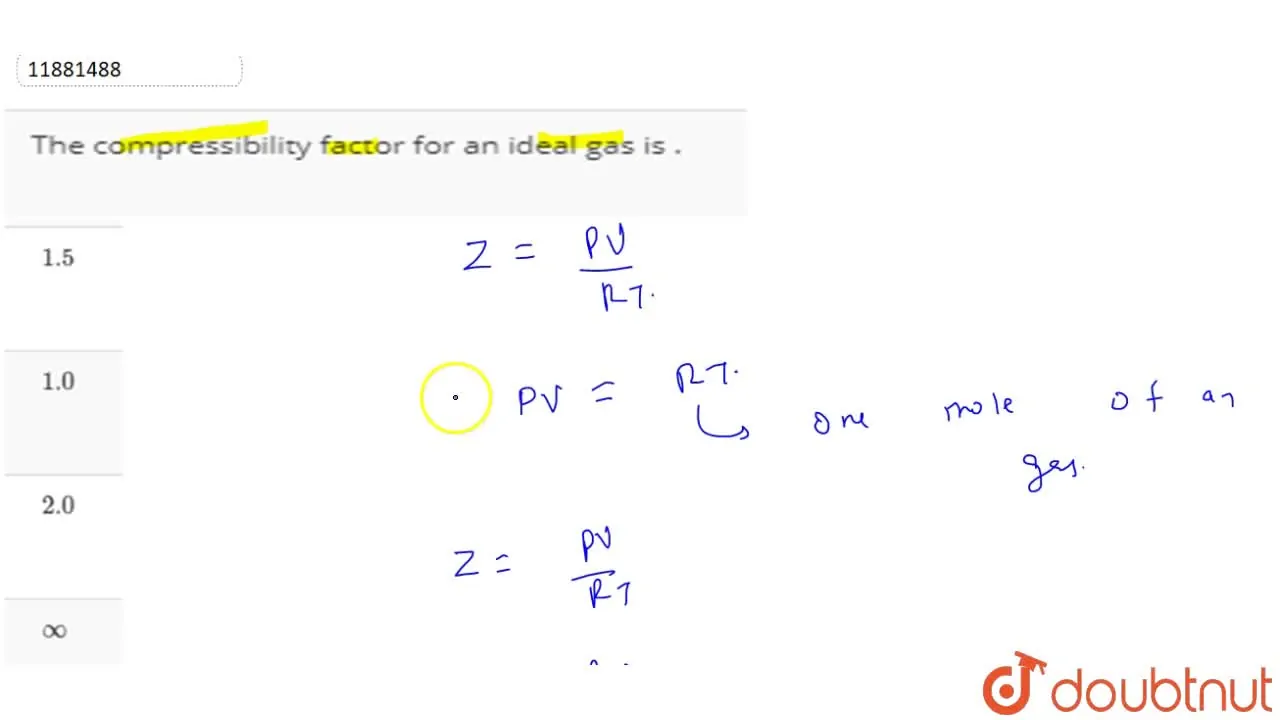
The compressibility factor for an ideal gas is .
The compressibility factor Z for the gas is given by

At Boyle's temperature , compressibility factor Z for a real gas is

Statement-1 is correct, Statement-2 is incorrect.
Why there is different between the value of compressibility factor at critical point between real and ideal gas? - Quora
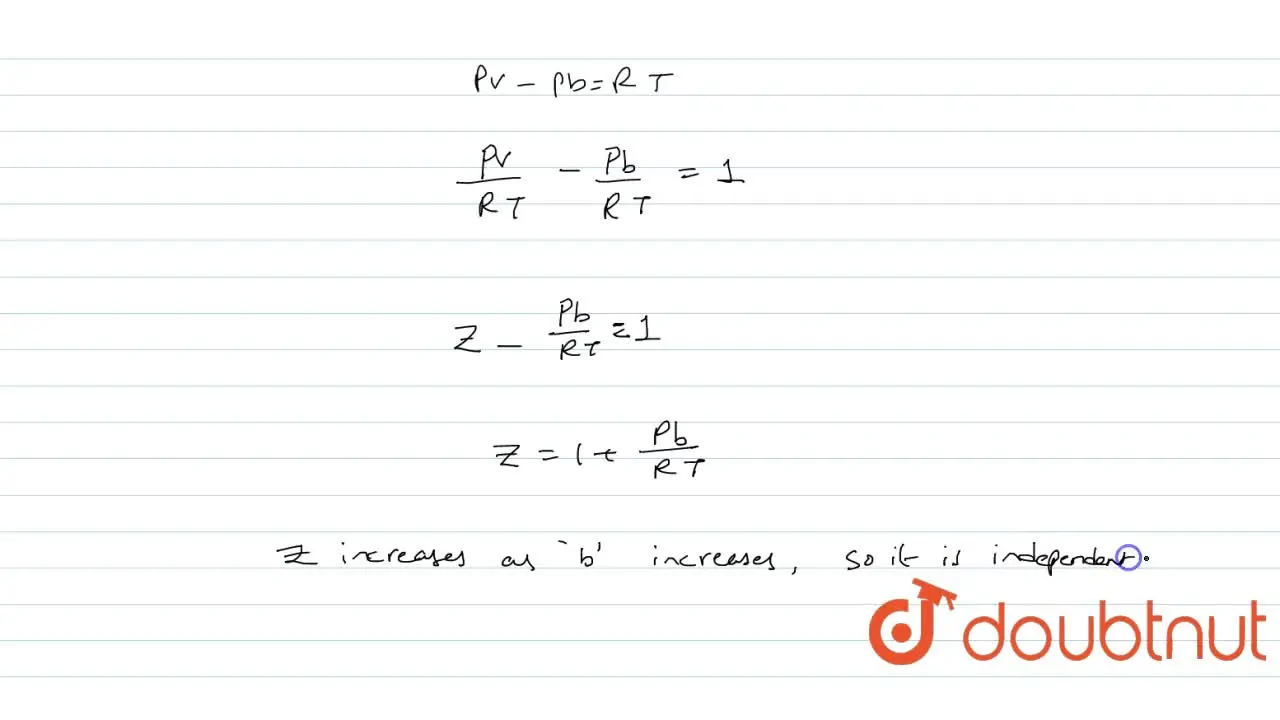
The rise is compressibility factor (Z) with increasing pressure of a g










