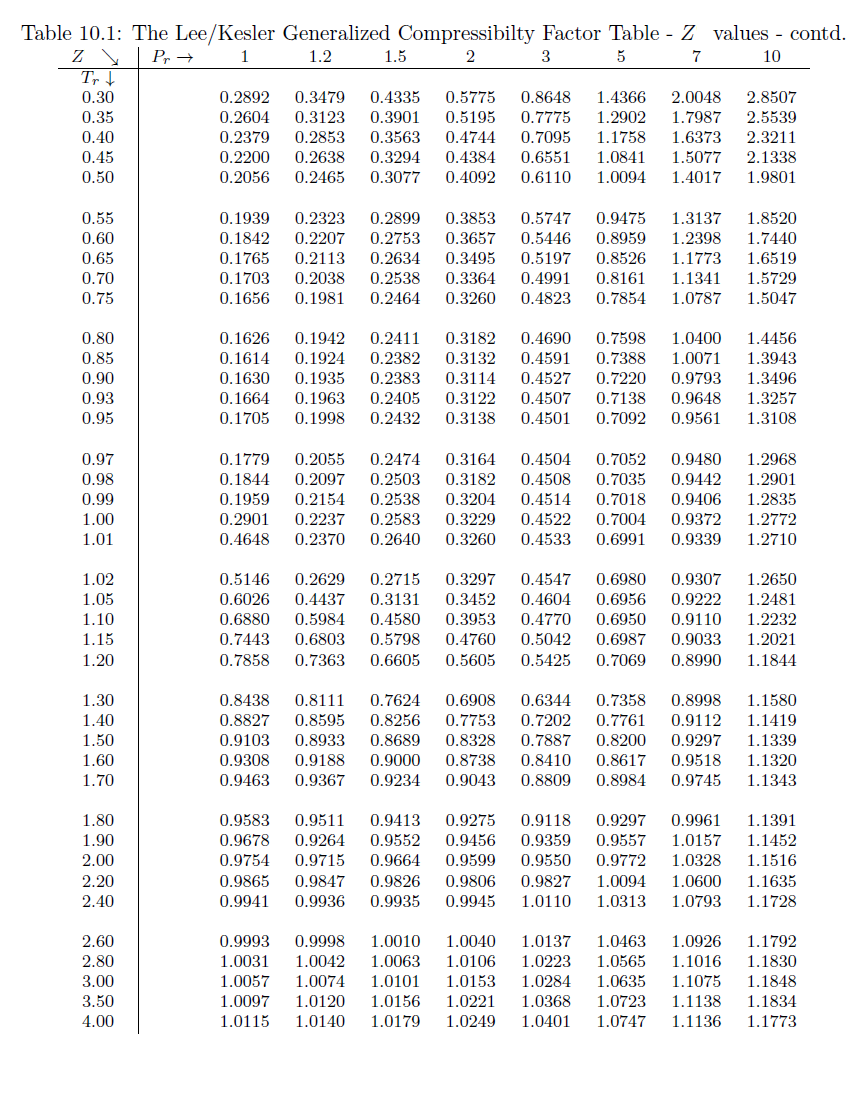
Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an

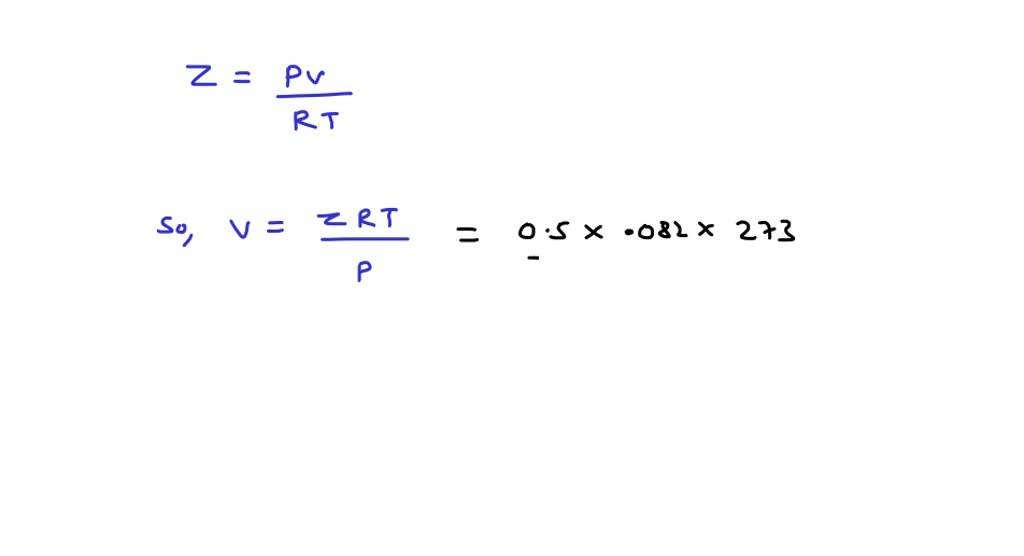
⏩SOLVED:Compressibility factor for 1 mol of a van der Waals gas at…

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
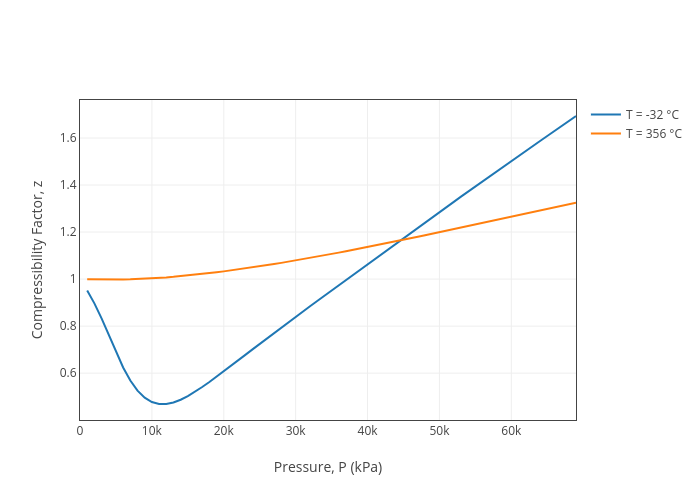
Energies, Free Full-Text

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Notes On Gaseous State (BSc and Integrated Standard For all Concerned Entrance Examination)
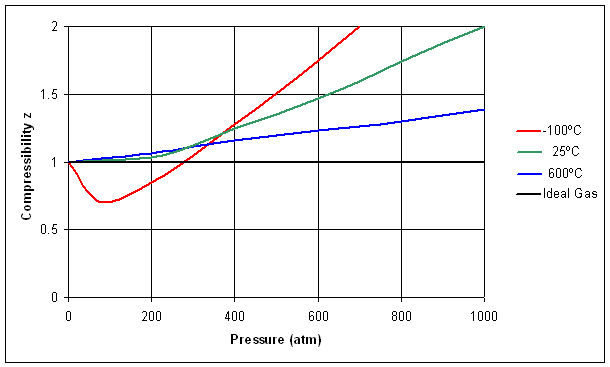
Gas Laws – First Year General Chemistry
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

SOLVED: Hey guys, please help me friends. Choose the correct answer, don't say wrong answers. Select correct statement/s regarding compressibility factor Z of a gas: Z for an ideal gas is independent

SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der