The value of compression factor at the critical state of a vander
The value of compression factor at the critical state of a vander waals gas is

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor - Wikipedia

Gaseous State.pdf - Chemistry - Notes - Teachmint
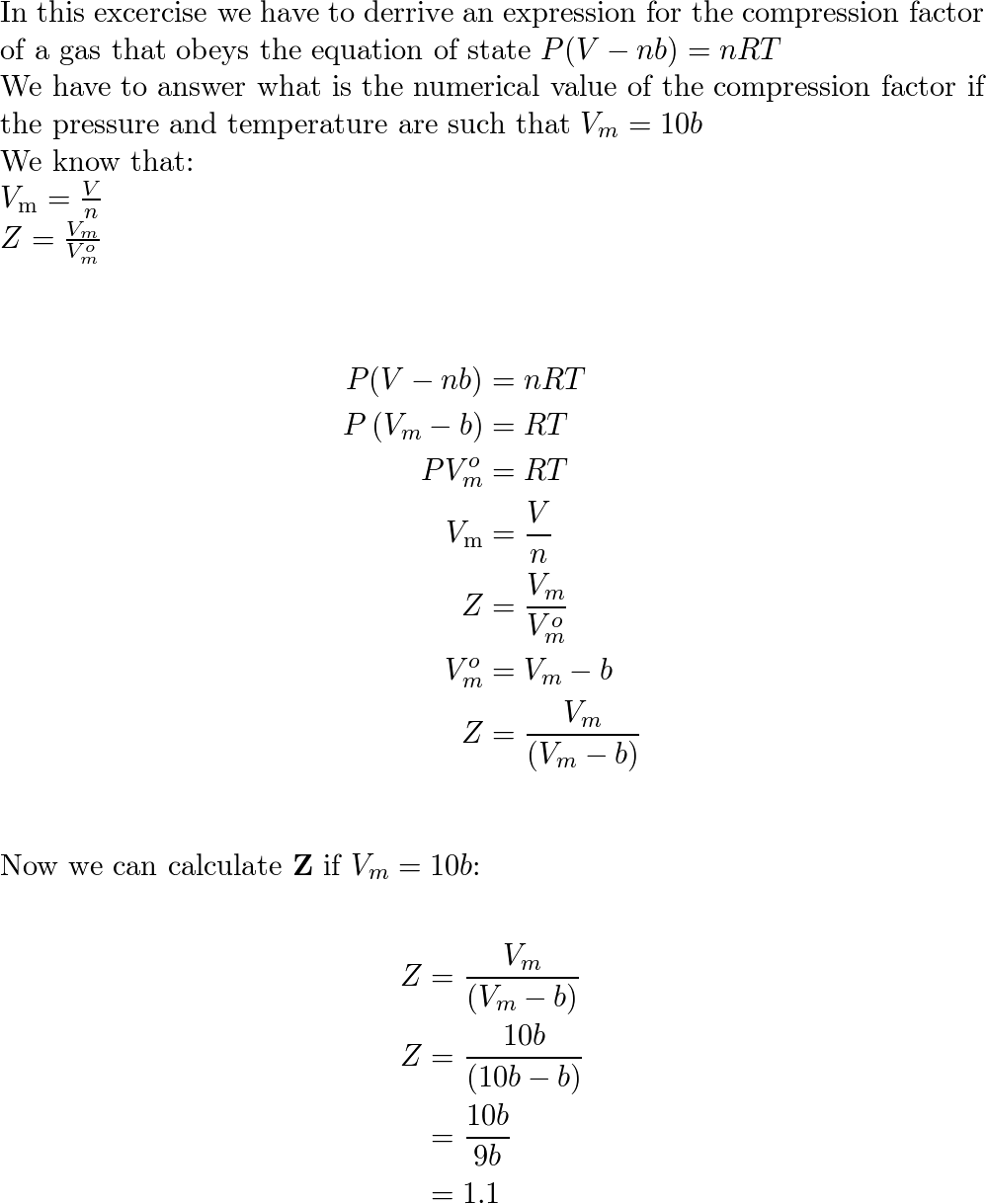
Derive an expression for the compression factor of a gas tha

1. A Choose the correct option(s) A) At low pressure (nearly 1 atm), compressibility factor H, gas is greater than 1 273 K. VB) Compressibility factor a vander Waal's gas its critical

Compressibility Factor Calculator - File Exchange - MATLAB Central

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Critical constants and parameters of the PRSV equation for hydrogen

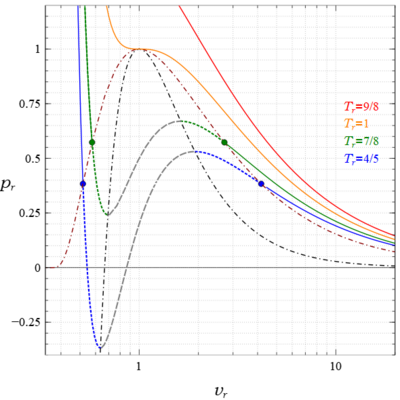
Liquid-vapour saturation curve and Γ < 0 region (shaded region) for a

Gaseous State.pdf - Chemistry - Notes - Teachmint

For one mole of a gas, under critical conditions, the compressibility factor is
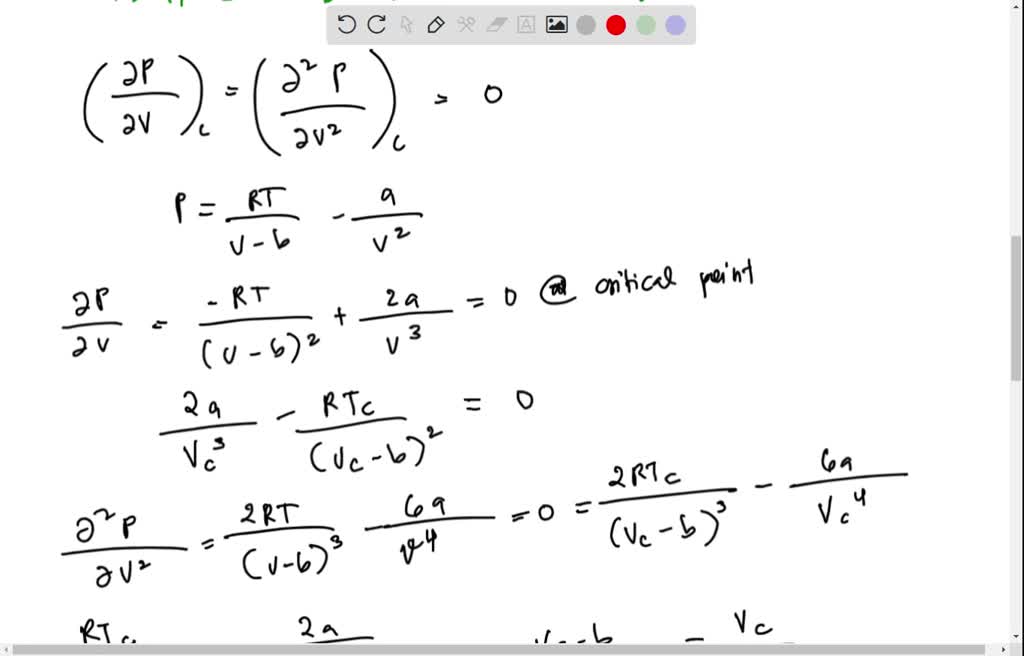
SOLVED: 1) Estimate/ Calculate the critical constants (pc, Vc, and Tc) for a gas molecule whose van der Waals parameters are a = 1.32 atm dm^6 mol^-2 and b = 0.0436 dm^3

Solved (a) The van der Waals model of liquid-gas systems

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
Van der Waals equation - Wikipedia



:max_bytes(150000):strip_icc()/sme-small-to-medium-enterprise-definition-2947962_final-65f516d722e44beaa0ea8d7c15a07c69.png)

