The correct order of increasing bond length of \( \mathrm{C
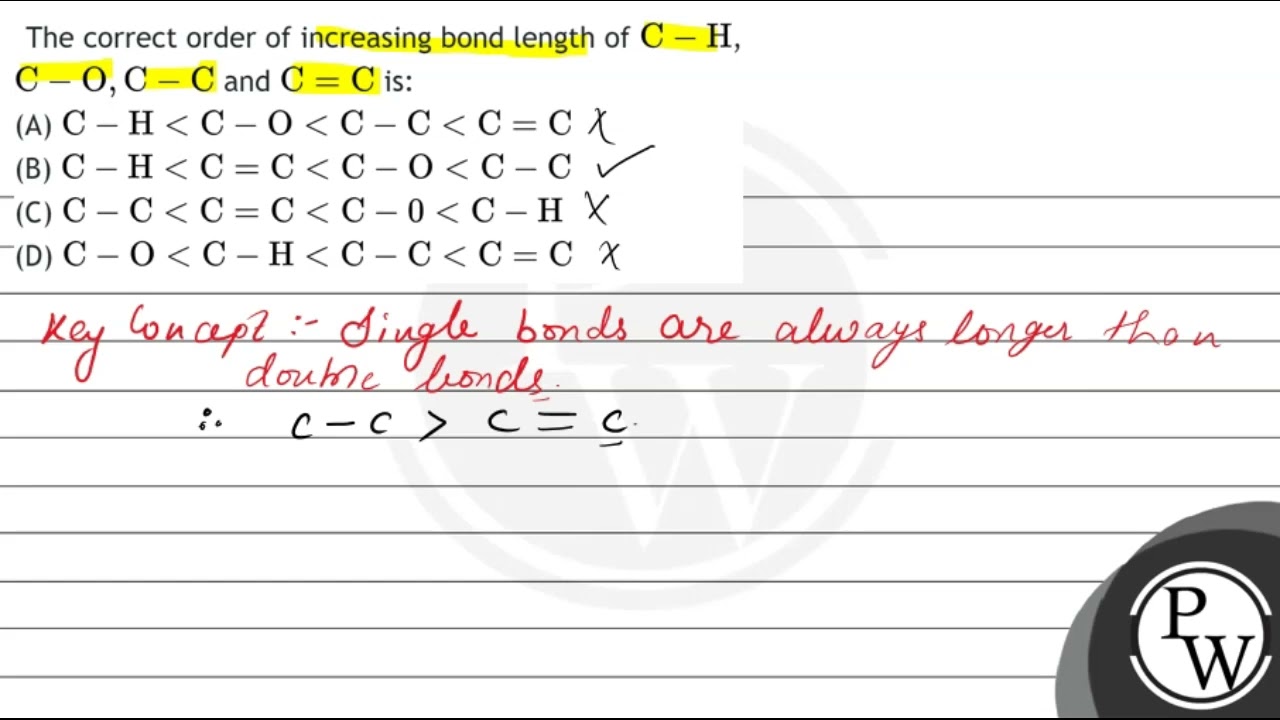
The correct order of increasing bond length of \( \mathrm{C}-\mathrm{H} \), \( \mathrm{C}-\mathrm{O}, \mathrm{C}-\mathrm{C} \) and \( \mathrm{C}=\mathrm{C} \
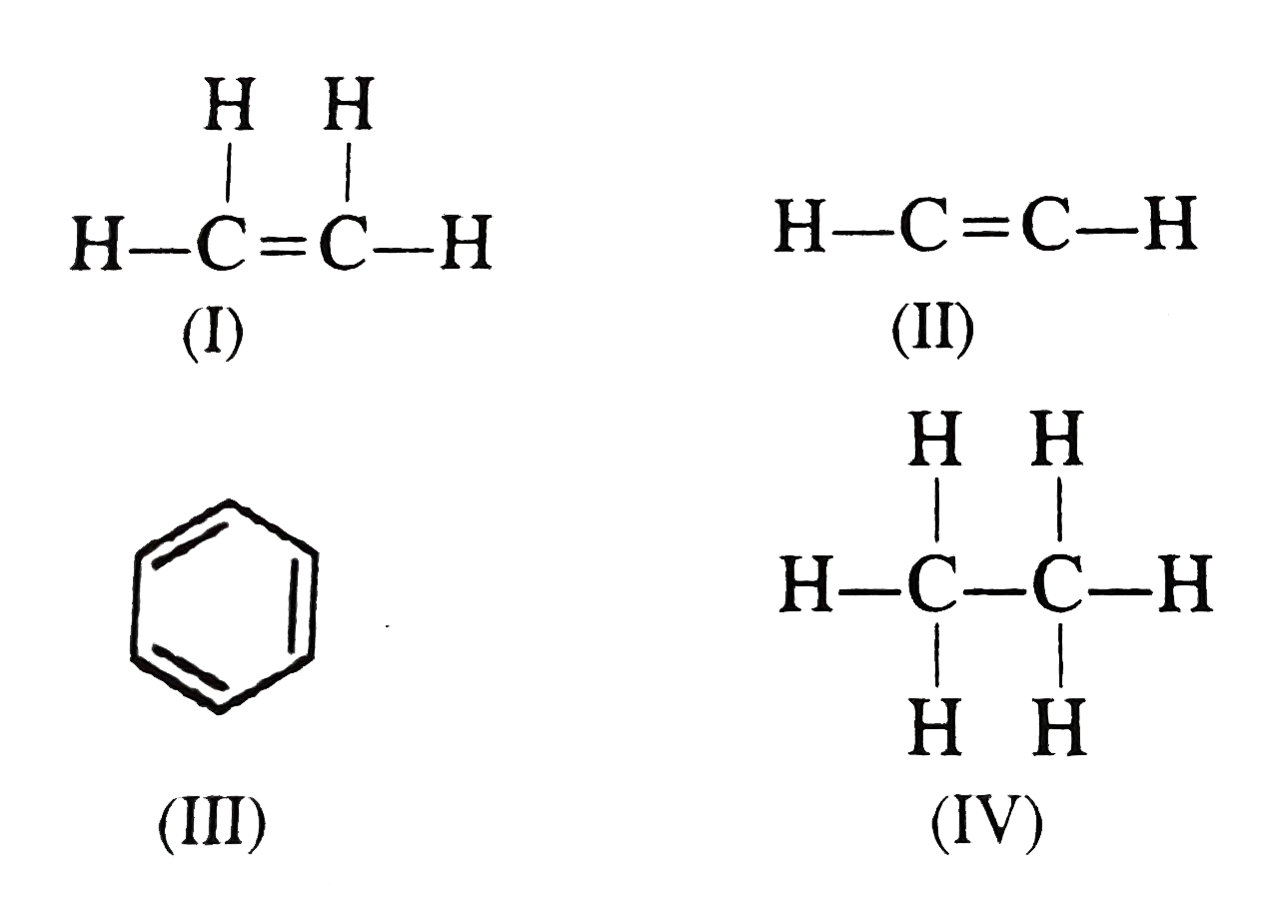
Decreasing order of C-C bond length is (I) C2H4 (II) C2H2 (III) C6 H6

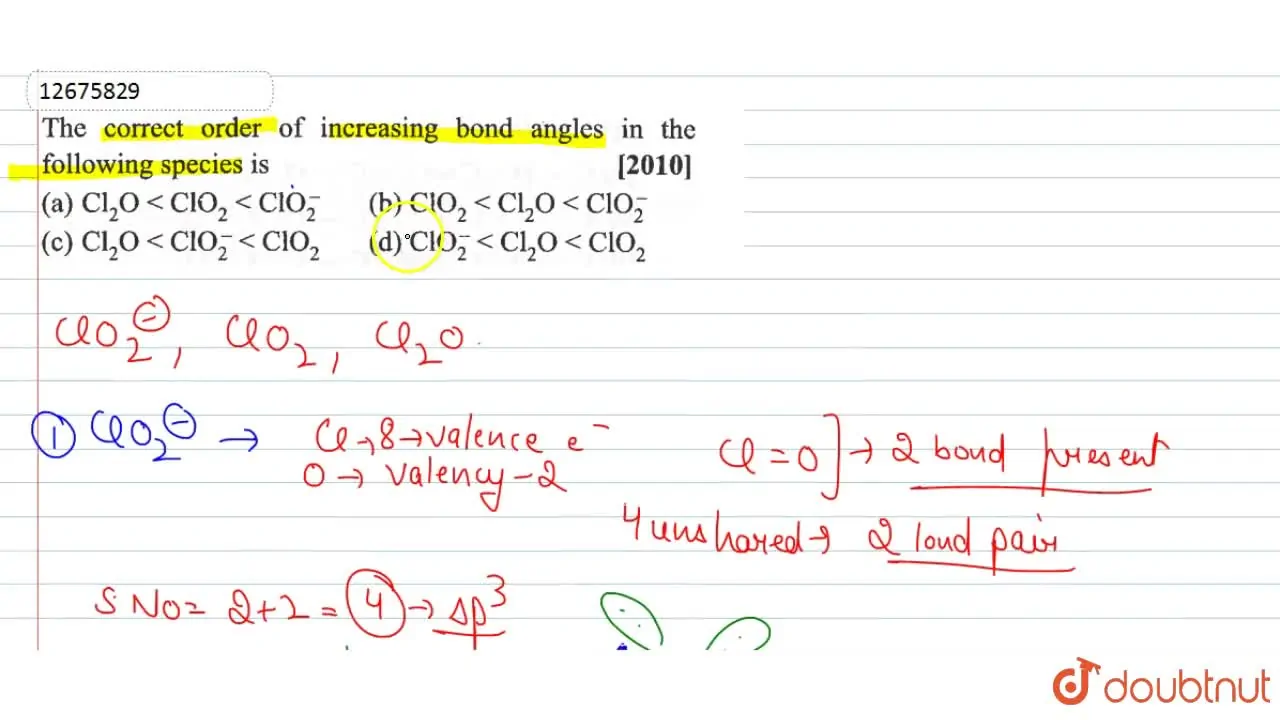
The correct order of increasing bond angle in the following species is

OpenKIM · EAM Dynamo MendelevKramerOtt 2009 CuZr MO_600021860456_005 MO_600021860456 · Interatomic Potentials and Force Fields

The correct order of increasing bond length of C–H, C–O, C–C and C=C is - NEETLab

Arrange the given species in increasing order of 0–O bond length H2O2, KO , Na2O2, 02 (1) I< III < IV <

The correct order of increasing bond length of mathrm{C}-mathrm{H}, mathrm{C }-mathrm{O}, mathrm{C}-mathrm{C} and mathrm{C}=mathrm{C} is (1) mathrm{C}- mathrm{H}







