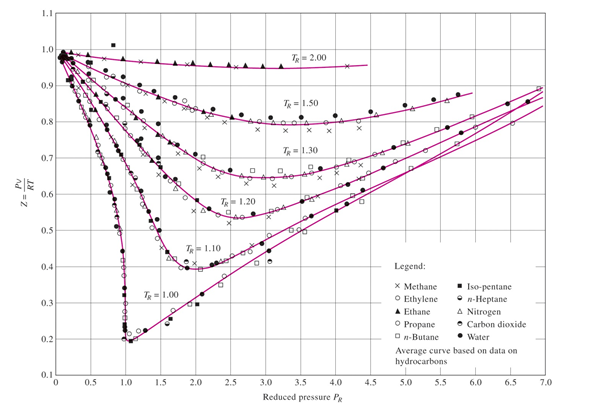
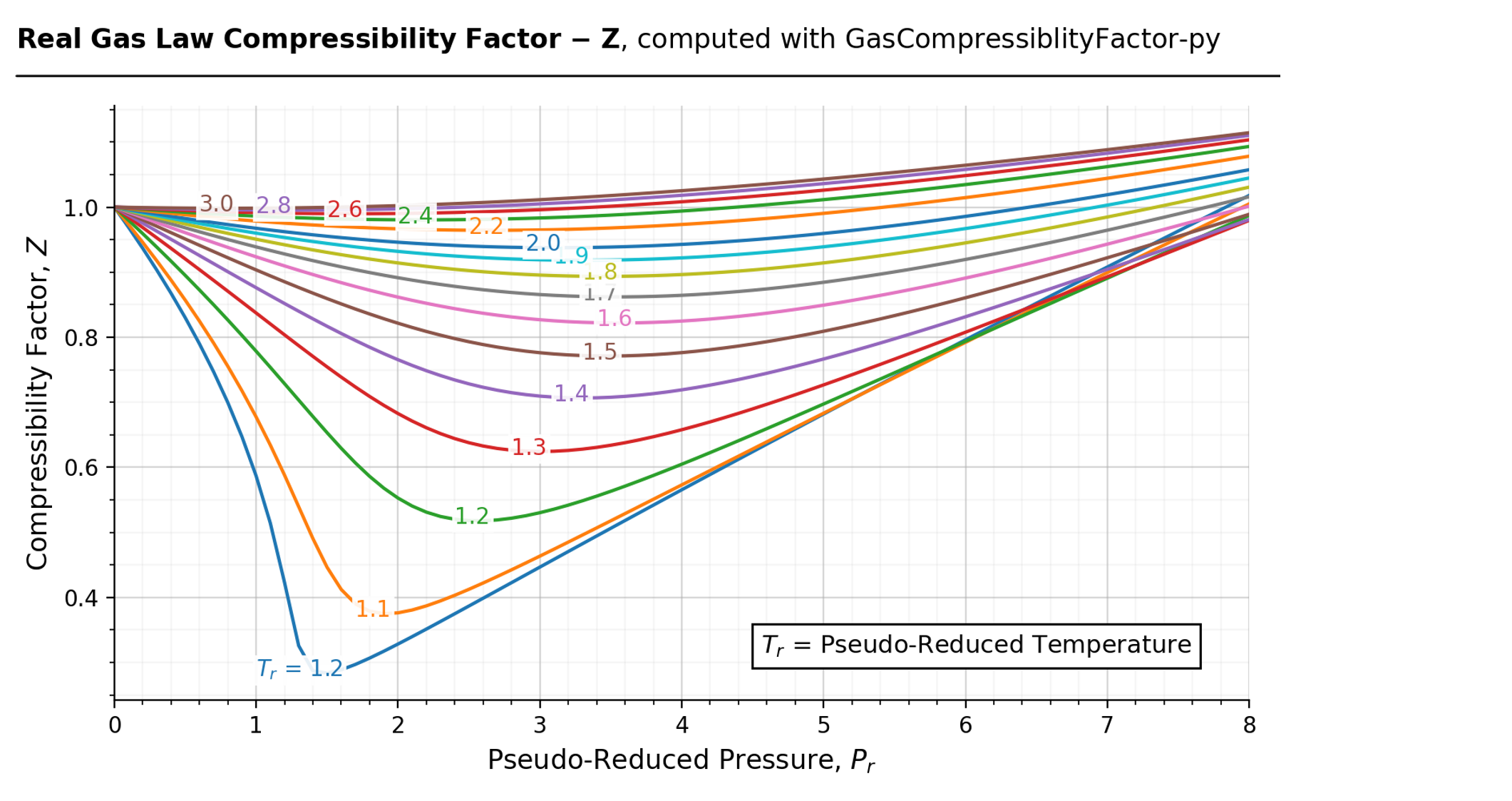
Solved QUESTION 3 Determine the compressibility

Solved] Compute the compressibility of acetone at 510 K and 60 bar using
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
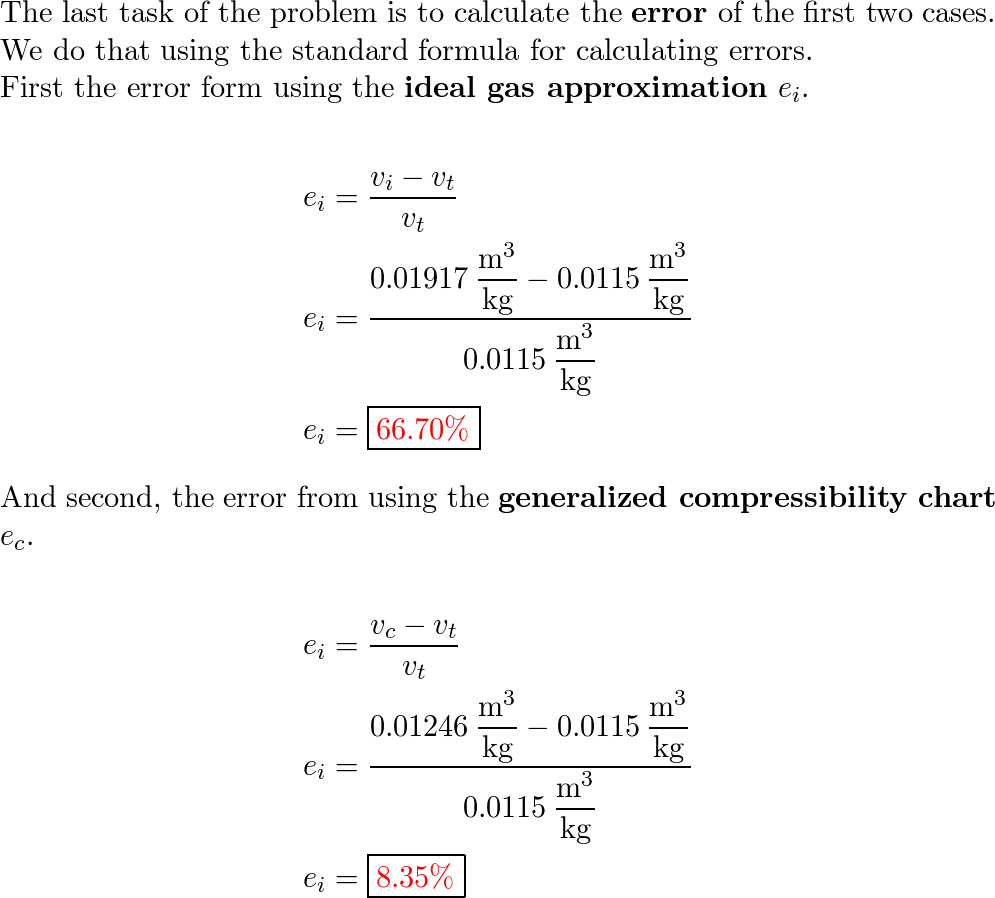
Determine the specific volume of superheated water vapor at

Density of van der Waals' gas 500 K and 1.0 atm was found to be 0.8 kg/m'. Also gas was found to effuse 1.37 times slower than oxygen under identical condition. Determine

The compressibility factor N_2 -50^oC and 800 atm pressure is 1.95. The mole of N_2 required to fill up a balloon of 100 L capacity are:2.24times 10^32.24times 10^22.2422.4
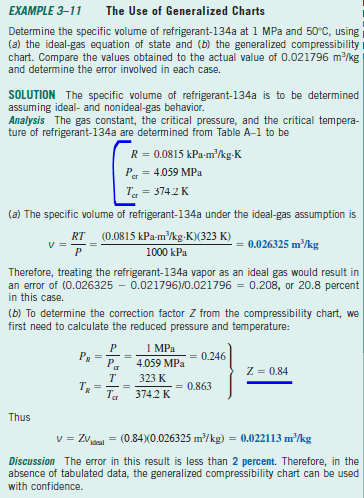
Solved Subject: Applied Thermodynamics Book: Cengel 8th

How to calculate compressibility factor for gas mixtures in gas cylinder?
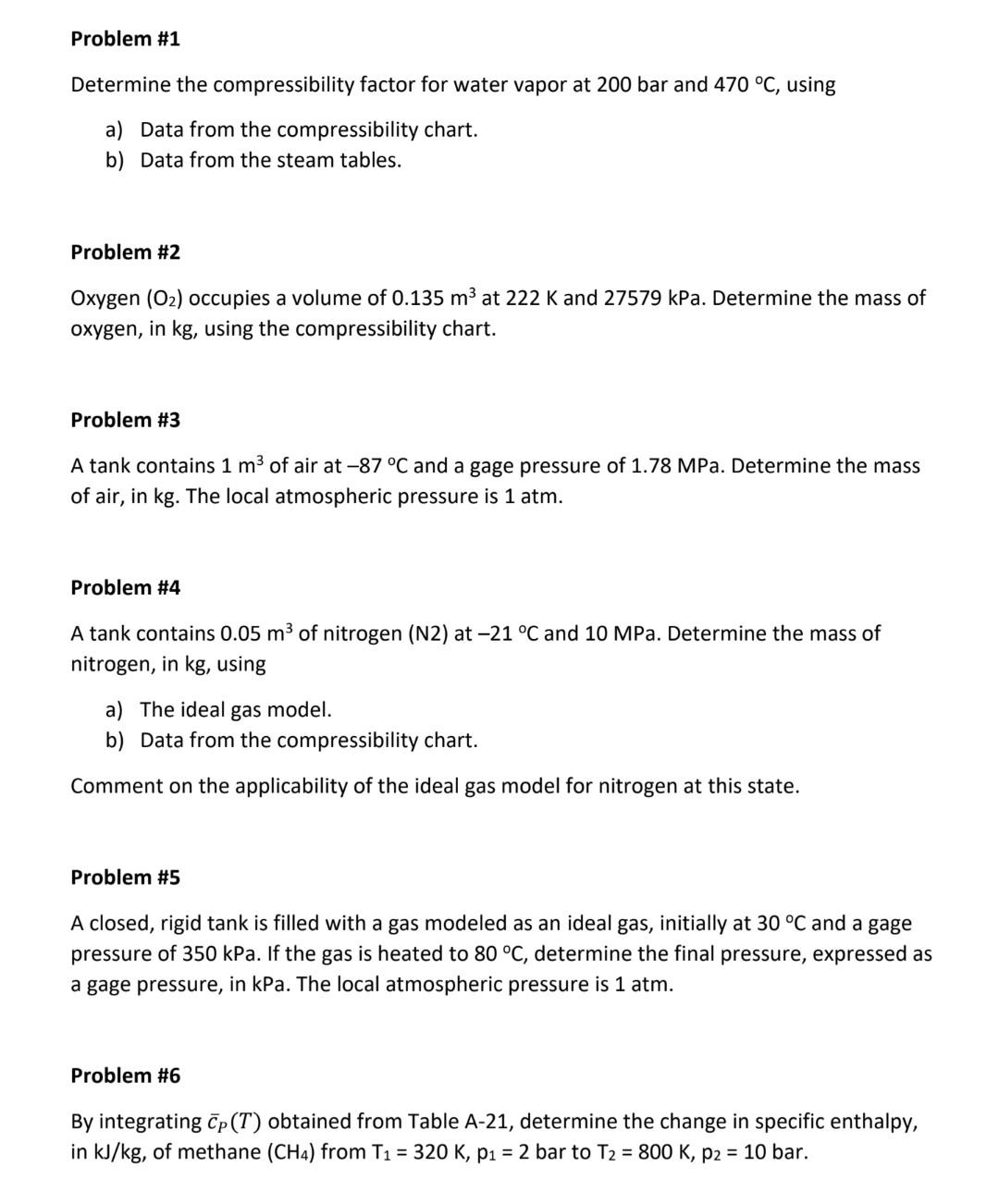
Solved Problem #1 Determine the compressibility factor for
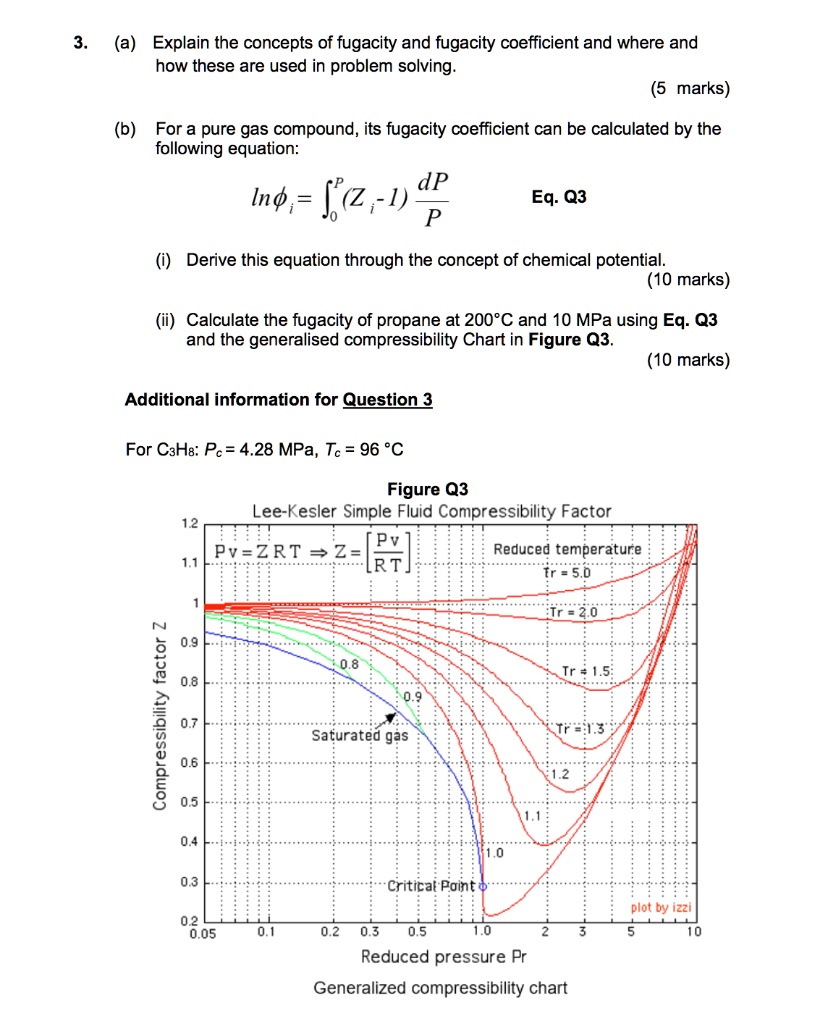
SOLVED: Thermodynamics Question 3: (a) Explain the concepts of fugacity and fugacity coefficient and where and how these are used in problem solving. (5 marks) (b) For a pure gas compound, its
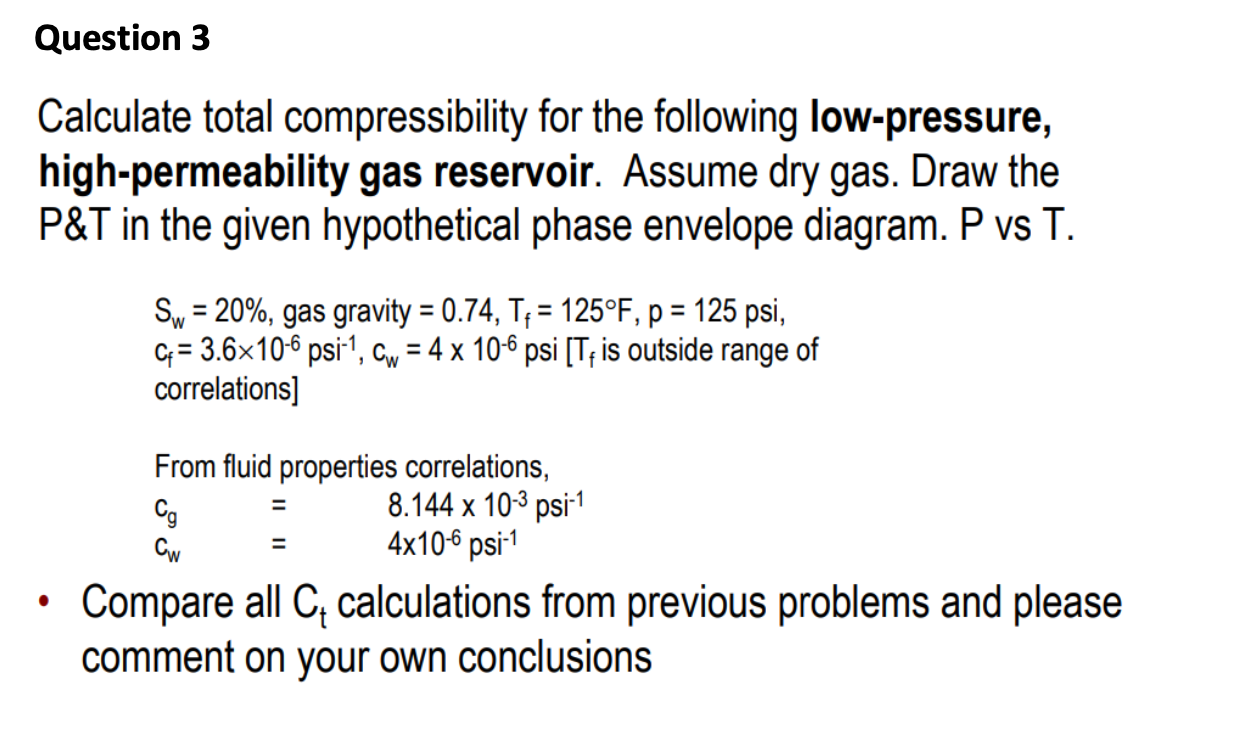
Solved Question 3 Calculate total compressibility for the
SOLVED: Problem 3 (25 points) Determine the specific volume of superheated water vapor at 10 MPa and 325*C, using (a) the ideal-gas equation, (b) the generalized compressibility chart; and (c) the steam