Microbiological Media Management - SOP & Guideline - Pharma Beginners

Description
Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.

Microbiology Laboratory procedures by GMP SOP - Issuu

Suitability of Microbial Count Method & its SOP

Microbial Culture Media Preparation

Entry and Exit of Micro Lab Sop, PDF, Clothing

How To Establish Growth Promotion Tests For Pharmaceutical Culture Media

Pharmaceutical Microbiology Resources: Microbiological Culture

Method Changes for Bacterial Endotoxins Testing (BET): Steps to

Entry and Exit of Micro Lab Sop, PDF, Clothing
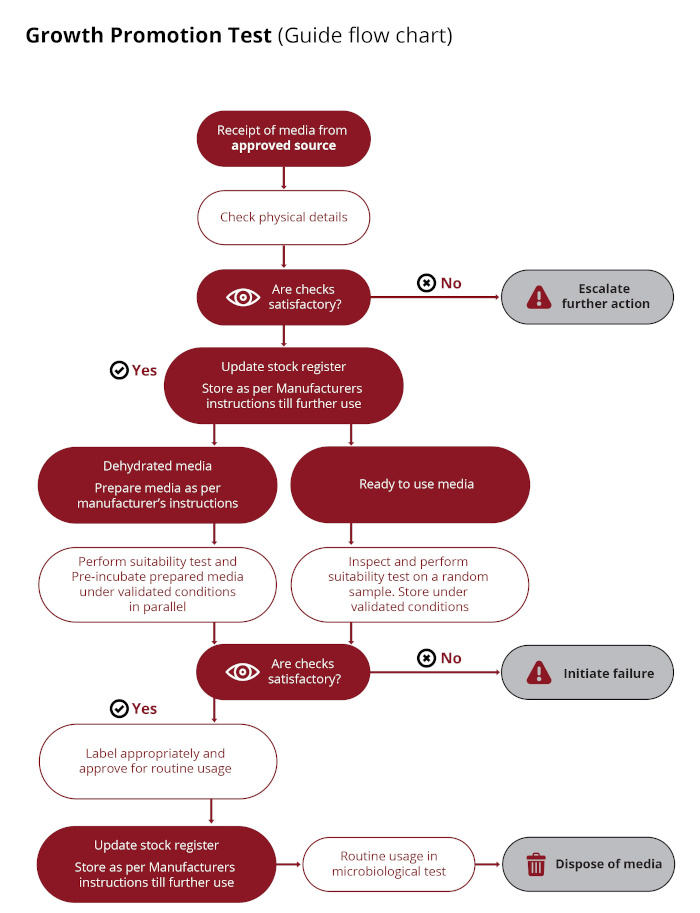
The importance of growth promotion testing
Related products
$ 11.99USD
Score 4.9(369)
In stock
Continue to book
$ 11.99USD
Score 4.9(369)
In stock
Continue to book
©2018-2024, followfire.info, Inc. or its affiliates