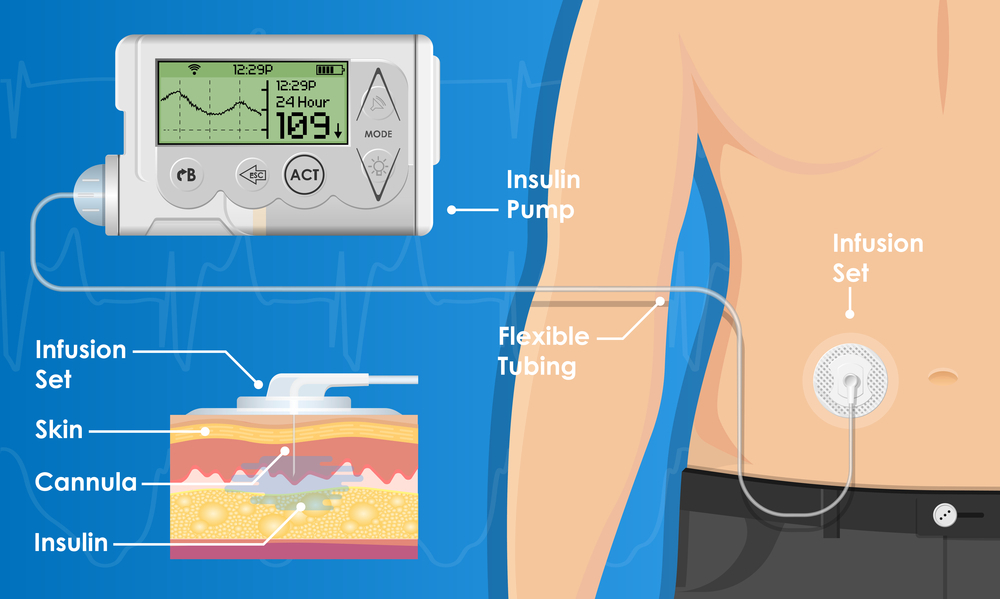
FDA warns on cybersecurity risk with Medtronic insulin pump
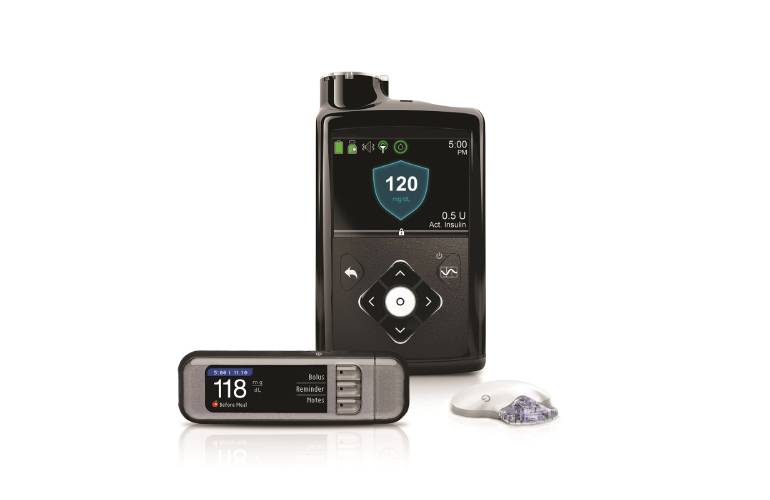
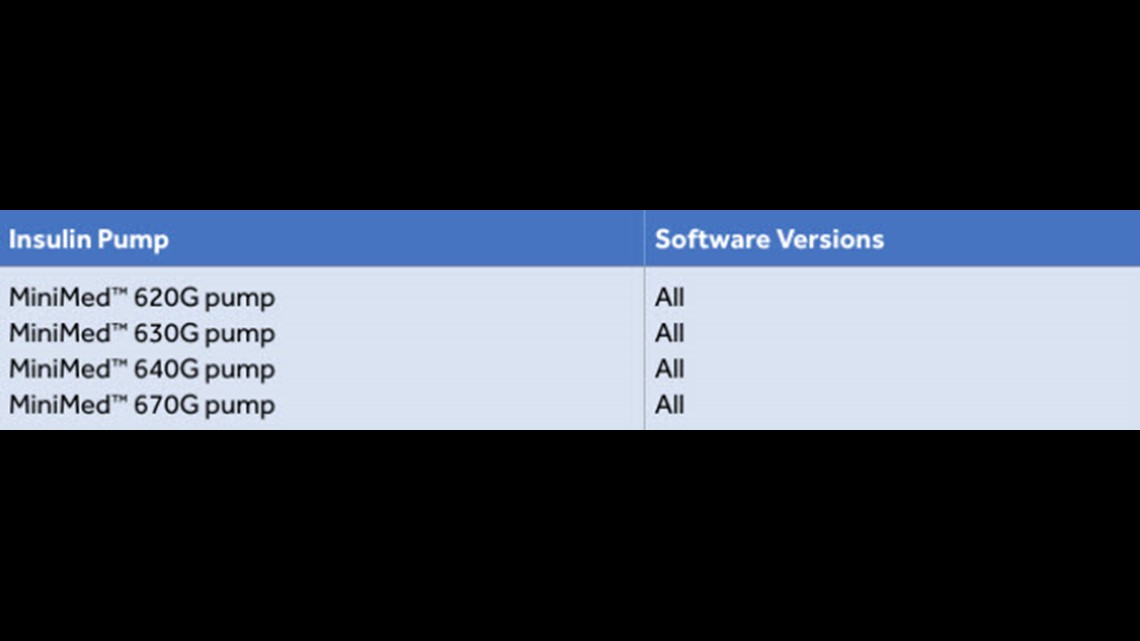
FDA warns patients and health care providers about potential cybersecurity concerns with certain Medtronic insulin pumps

FDA Issues Recall For Medtronic mHealth Devices Over Hacking Concerns

FDA warns of cybersecurity risk with certain Medtronic insulin pumps, ET HealthWorld
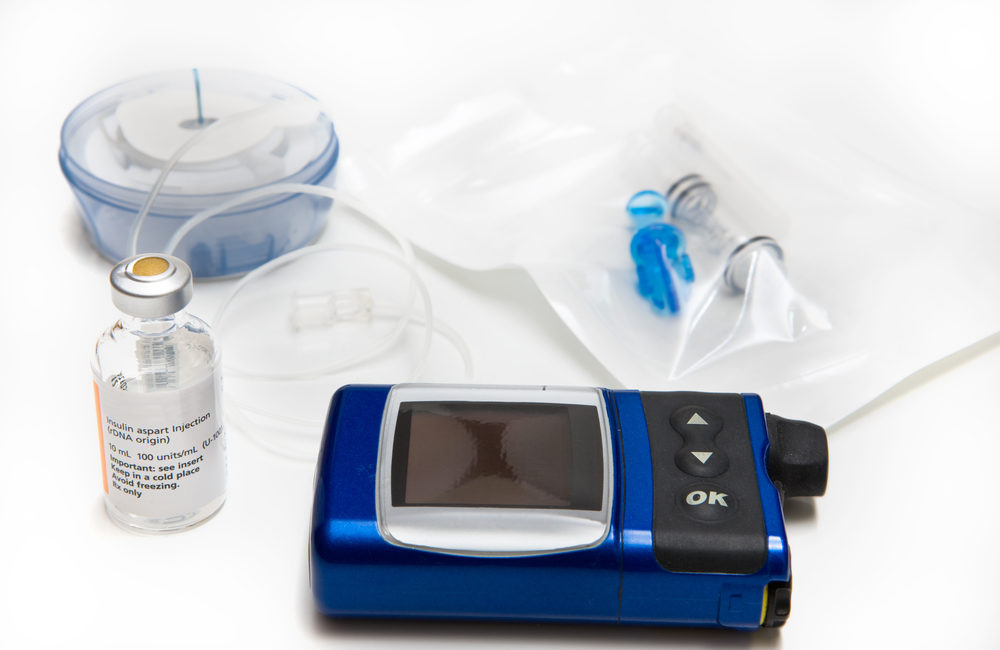
FDA Recalls Medtronic Insulin Pumps Due to Hacker Risk - Drug And Device Watch

U.S. health regulator flags concerns at Medtronic's diabetes business

Insulin Pump Recalled due to Cybersecurity Risks

FDA issues new alert on Medtronic insulin pump security

Blood Sugar: Drug regulator CDSCO warns of cyber security risk to certain Medtronic insulin pumps, ET HealthWorld

FDA warns of Medtronic's insulin pump hacking risk

Medtronic recalls MiniMed insulin pumps as FDA warns about hacking risk

Diabetes and the benefits, risks of personal health on the internet

Pre-2013 Medtronic insulin pumps could be vulnerable to hacking