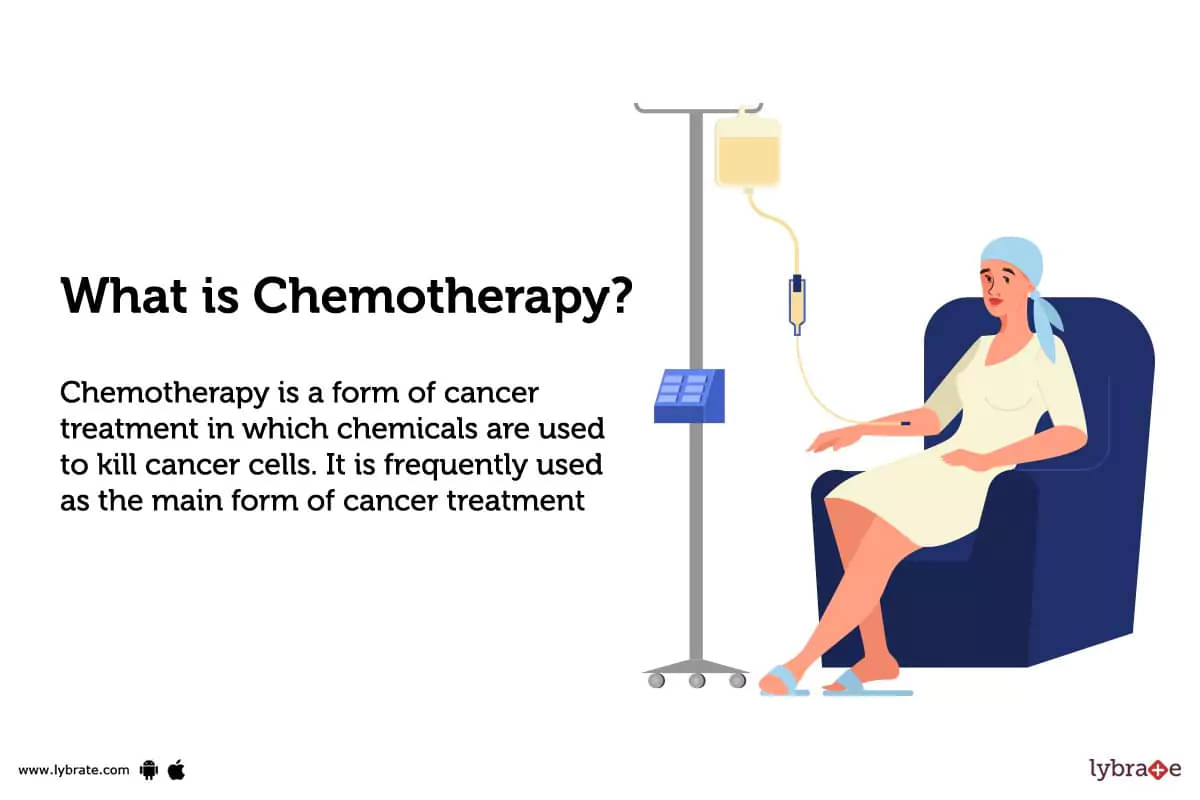
How serious is FDA warning about revolutionary blood-cancer treatment?— Harvard Gazette
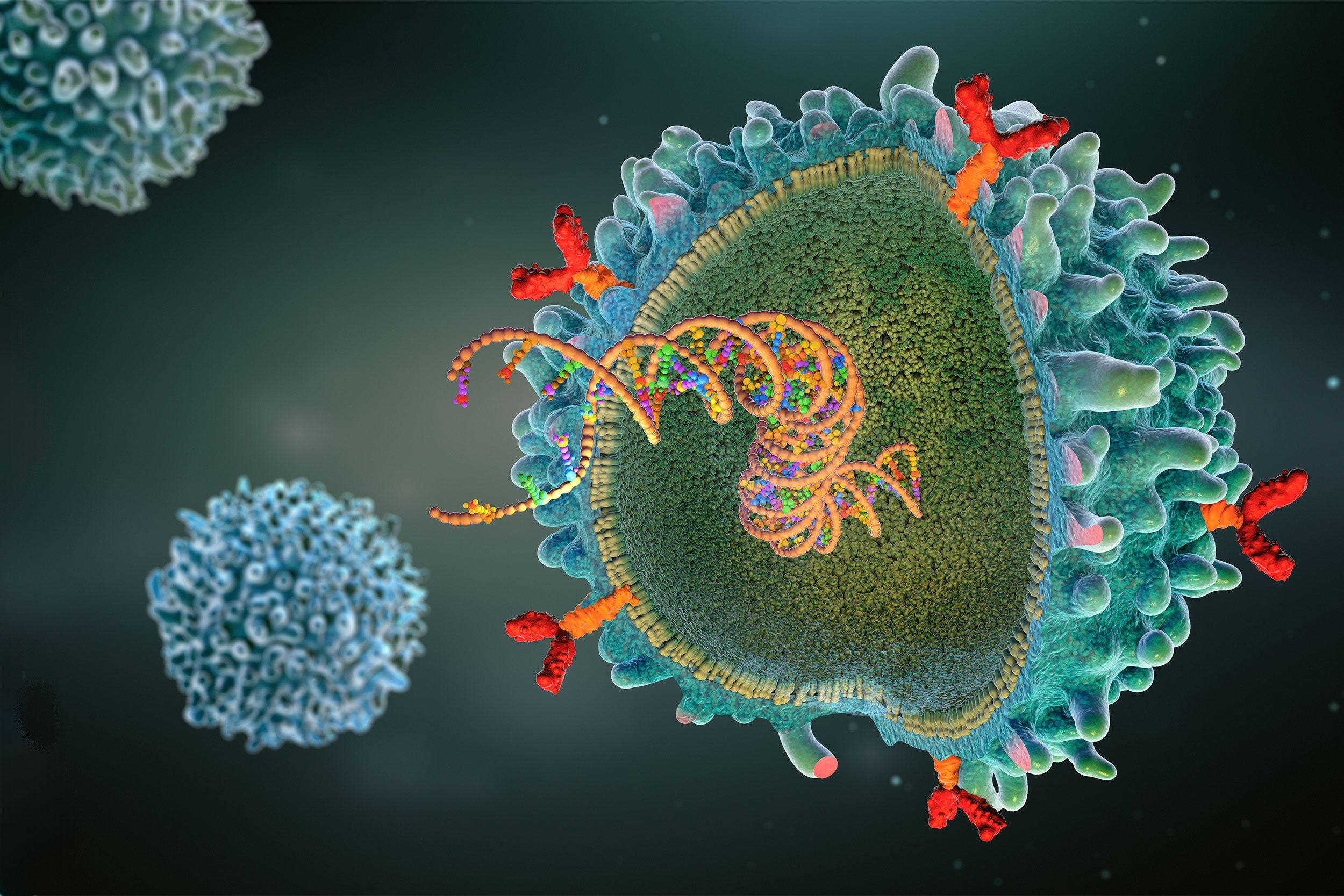
Dana-Farber Cancer Institute researcher details promise, peril of CAR T-cell therapy, which enlists body’s immune system to fight disease.

Susan B. Nichols en LinkedIn: FDA to back accelerated approval pathway for gene therapies (NASDAQ:SRPT)

Jason King on LinkedIn: Home

Mark Flower on LinkedIn: Kite releases pivotal CAR T therapy Phase III study results

Ming Ni on LinkedIn: Secondary Cancers after Chimeric Antigen Receptor T-Cell Therapy

Jason King on LinkedIn: Cell and gene therapy manufacturing: the next generation of startups

Susan B. Nichols on LinkedIn: Intellia, Verve run clinical trials abroad amid FDA's 'cautious' approach…

Mark Flower on LinkedIn: Kite releases pivotal CAR T therapy Phase III study results

Ming Ni en LinkedIn: Linearized Plasmid Manufacture

Susan B. Nichols on LinkedIn: Sanofi pays Scribe $25M to access CRISPR tools, writing new chapter in its…

Jason King on LinkedIn: Order TESSA

Jason King on LinkedIn: Plasmids & Cell Lines

Natasha Hansjee en LinkedIn: Excited to be part of this panel, to learn and to engage!

Susan B. Nichols on LinkedIn: Bluebird submits sickle cell gene therapy to FDA, setting up race with…

James Wang, PhD on LinkedIn: Phase 1 trial to open into SC291, cell therapy for AAV

Byung-Soo Youn on LinkedIn: ~ Put your experimentations on rational thinking! / Osteoneurogen I used…