Applications for Medical Device Investigational Testing Authorizations Guidance Document

Applications for Medical Device Investigational Testing Authorizations Guidance Document

US FDA Pre-Market Notification - 510(k)

Regulatory oversight of genetic testing in Canada: Health Canada

What is Investigational New Drug Application?

Frontiers Advanced Therapy Medicinal Products' Translation in Europe: A Developers' Perspective

IND Application for Botanical Drug Products - The Johns Hopkins

Micromachines, Free Full-Text
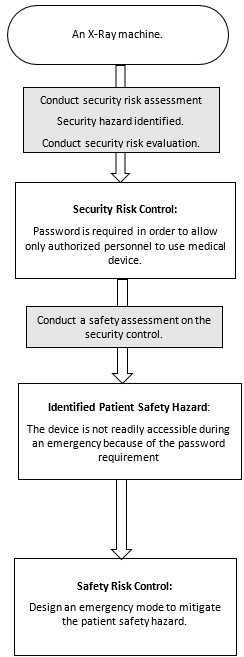
Guidance Document: Pre-market Requirements for Medical Device

Reporting Processes to Health Canada - ppt download

Noncovered Investigational Services - Tufts Health Plan

Current practices and reform proposals for the regulation of
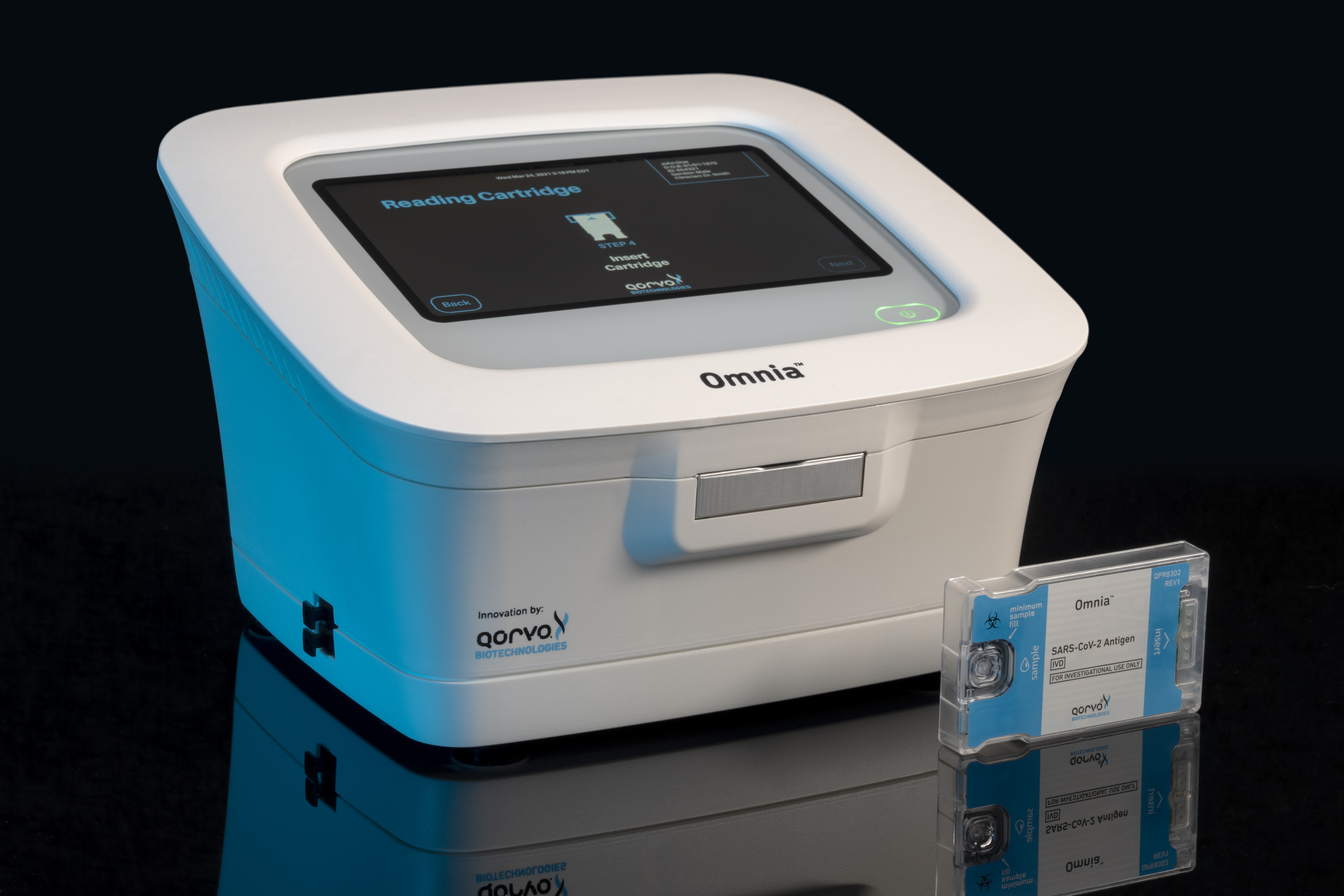
Qorvo Biotechnologies Receives FDA Emergency Use Authorization (EUA) for Rapid COVID-19 Antigen Testing at the Point of Care - Qorvo

Emerging Issues in Using Mobile Apps for Clinical Research - New York State Bar Association