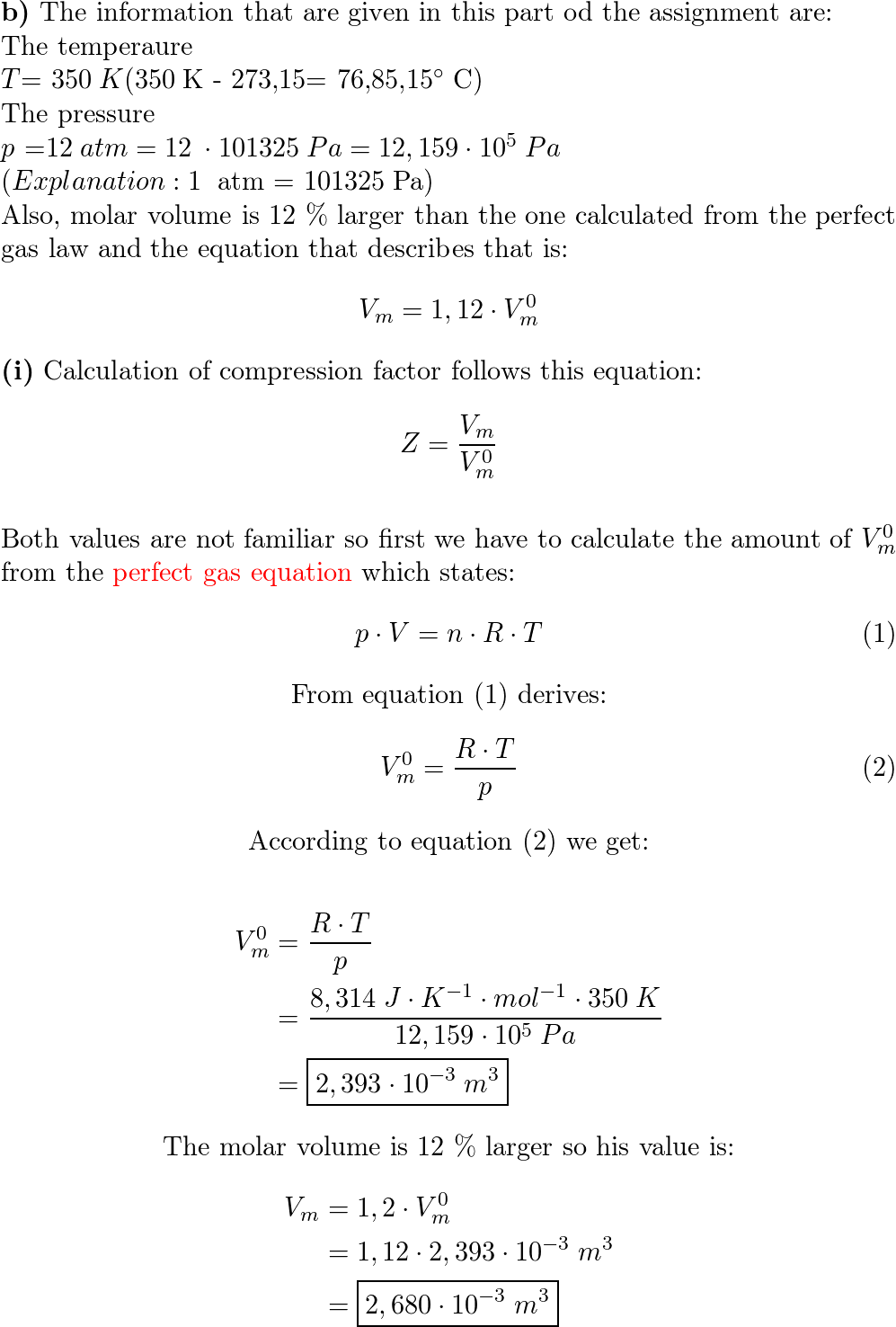
Solved RT B 2. The compressiblity factor for a gas is

Answer to Solved RT B 2. The compressiblity factor for a gas is
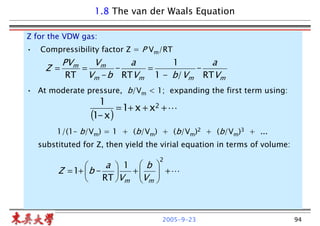
Real Gases and the Virial Equation

SOLUTION: Thermo - Studypool

Compressibility Factor Z Important Concepts and Tips for JEE Main

Compressibility factor (gases) - Knowino

Compressibility factor (z): real gases deviate from ideal behav-Turito

Real gases

Physical Chemistry The Compression Factor (Z) [w/1 example]

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

For an ideal gas, the value of compressibility factor `Z(=(pVm)/(RT))` is

Gas Compressibility - an overview
Solved] please help with this question There are regimes in which the

Gas Compressibility - an overview
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts