Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage
Description

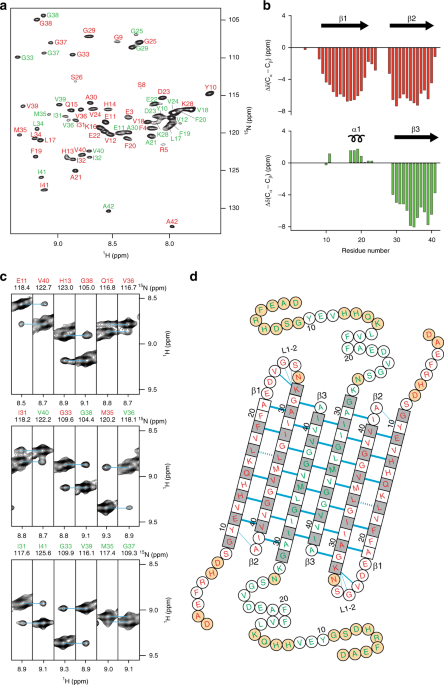
Molecular dynamics simulations reveal the importance of amyloid
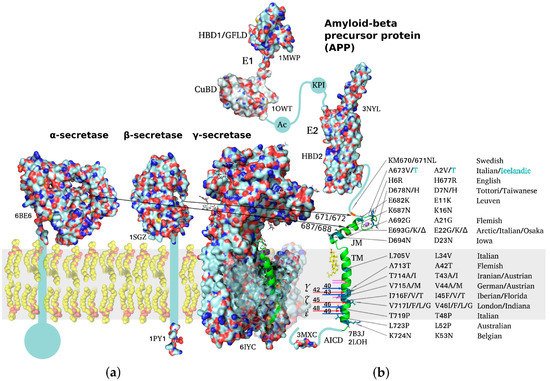
Aβ-Peptide Production and Conformational Behavior

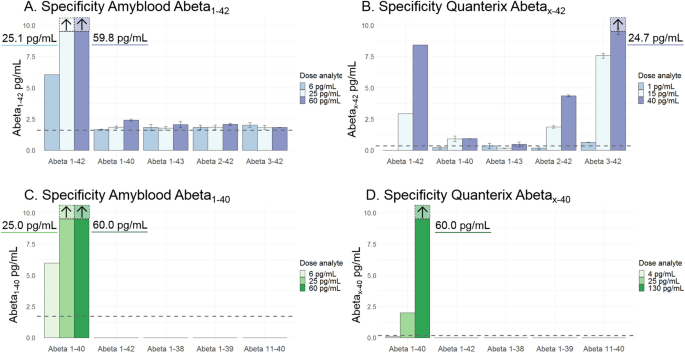
βPFOAβ(1-42) samples can be enriched in either tetramers or

Frontiers Purinergic signaling via P2X receptors and mechanisms

RCSB PDB - 6RHY: Structure of pore-forming amyloid-beta tetramers
Andres Arango

In vivo induction of membrane damage by β-amyloid peptide

Molecules, Free Full-Text

LinkedIn CovalX 페이지: Aβ(1-42) tetramer and octamer structures
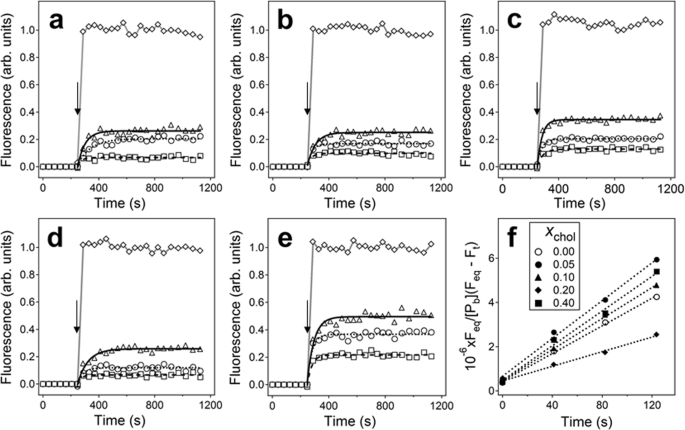
Structure of amyloid β25–35 in lipid environment and cholesterol

Aβ42 purification. (A) SDS-PAGE analysis of SUMO-Aβ42 fusion
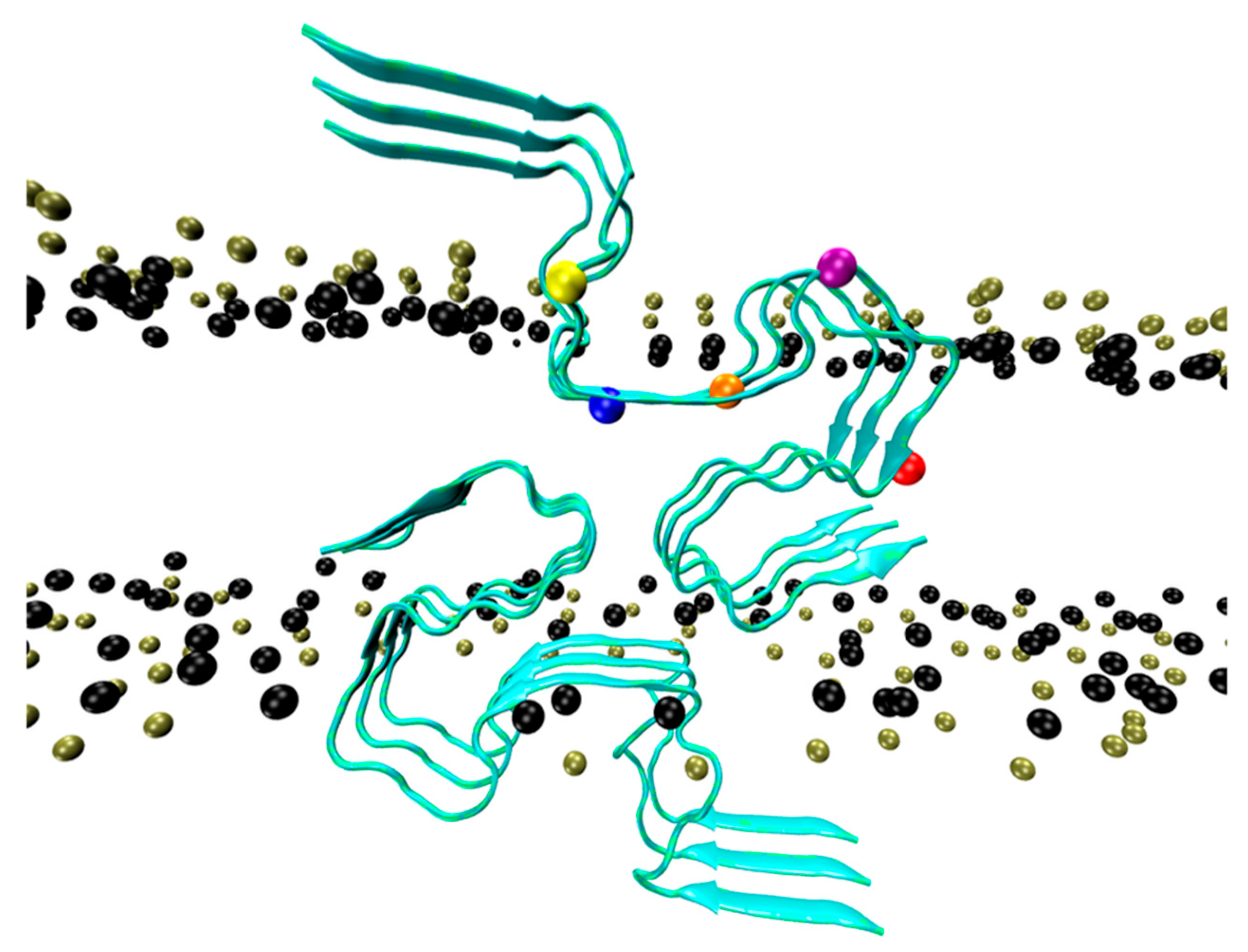
Molecules, Free Full-Text

Cholesterol Molecules Alter the Energy Landscape of Small Aβ1–42
Related products
$ 13.99USD
Score 4.9(144)
In stock
Continue to book
$ 13.99USD
Score 4.9(144)
In stock
Continue to book
©2018-2024, followfire.info, Inc. or its affiliates