200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95
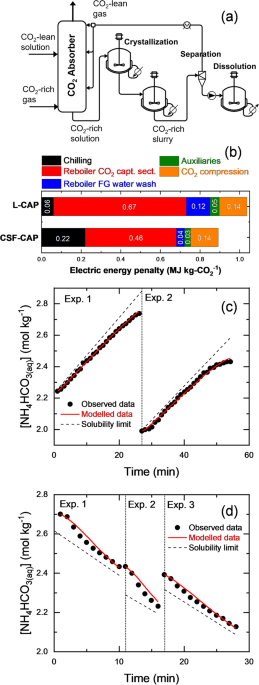
A review on chemical precipitation in carbon capture, utilization and storage, Sustainable Environment Research
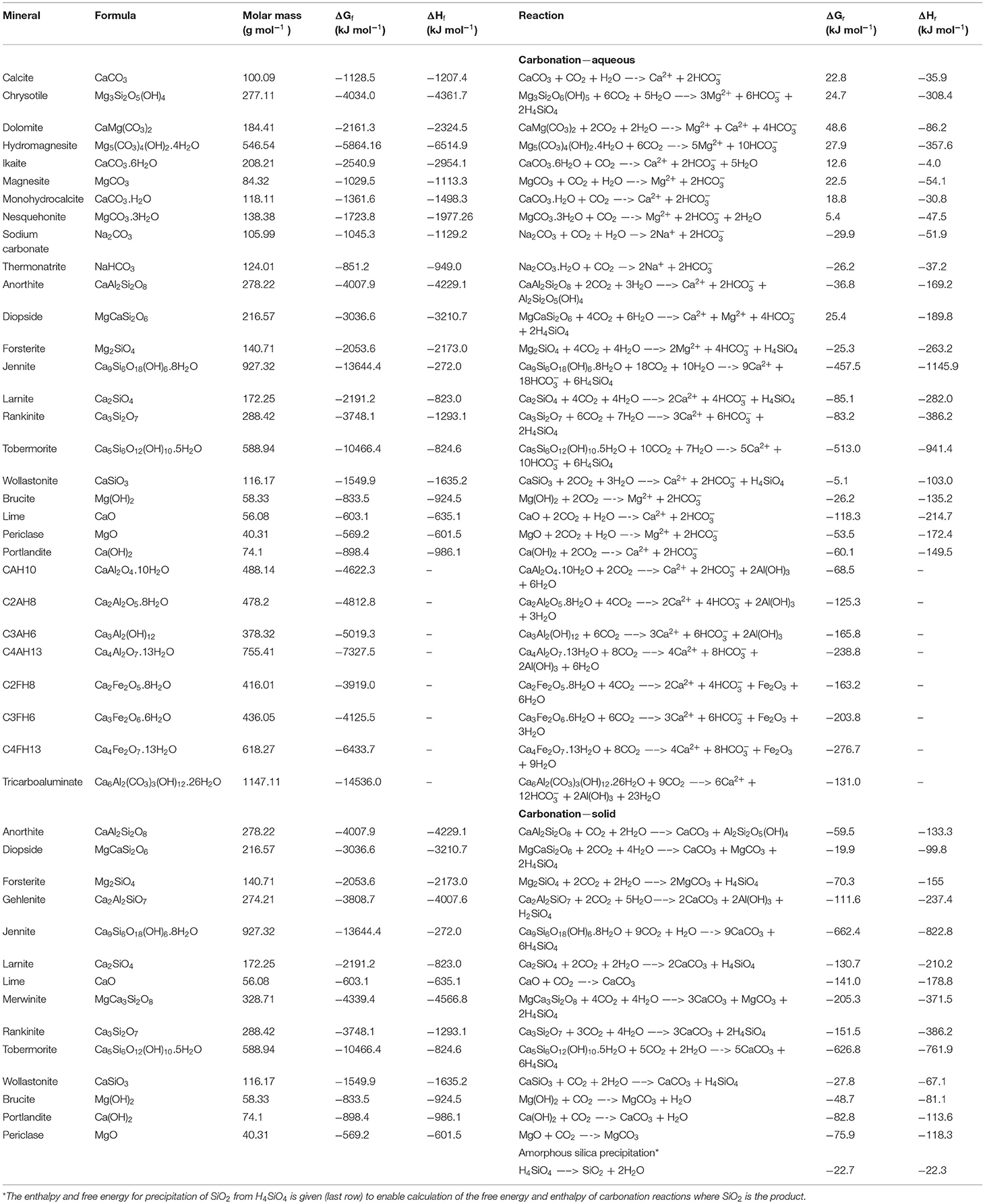
Frontiers Geochemical Negative Emissions Technologies: Part I. Review

Cement testing
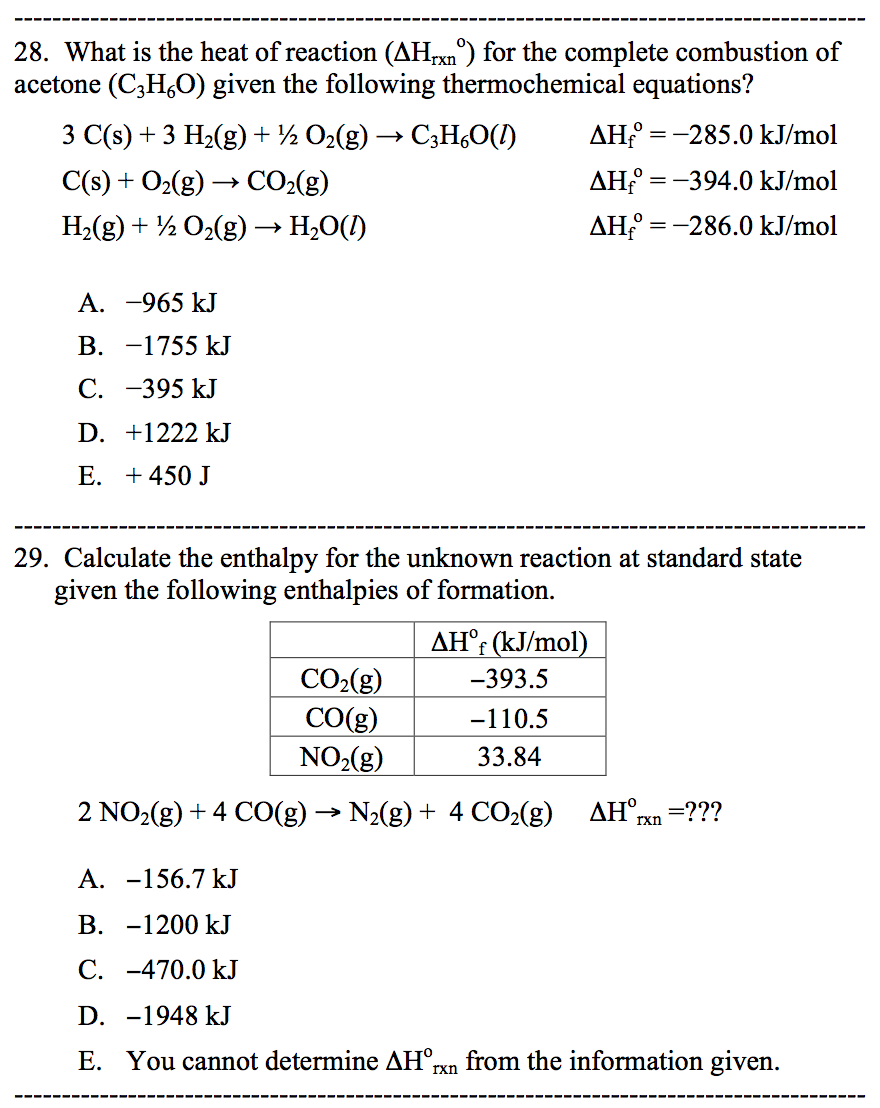
Solved Please help me solve the following questions below
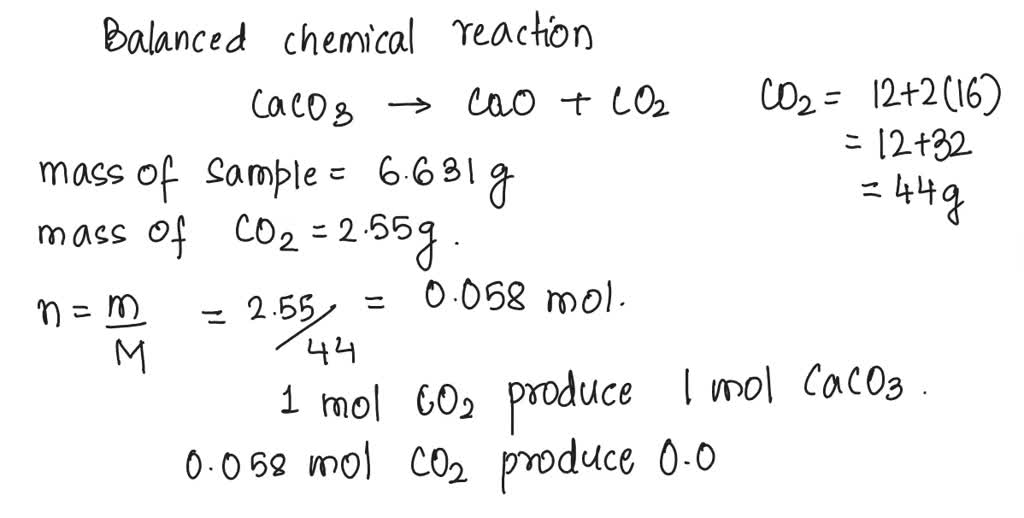
SOLVED: A sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide. CaCO3 (s) â†' 3 CaO(s) + CO2 (g) A 6.631

Modern inorganic chemistry

SOLVED: Calcium carbonate (limestone) decomposes when heated: CaCO3 CaO CO2 When 20.0g of calcium carbonate are decomposed, 11.2g of calcium oxide (lime), CaO, are formed. Calculate the mass of calcium oxide formed

Chemistry SampleSolvedArihant Chap 1 4, PDF, Mole (Unit)

Wu unswer the questions given below it: 150 ml of N HCI is required to react completely with 1.0 g of a sample of limestone. Calculate the percentage purity of CaCO3. (A)







